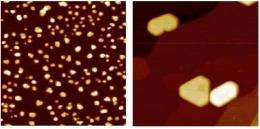
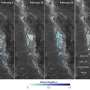
Physicists at the U.S. Department of Energy’s Ames Laboratory have discovered that rare-earth materials, such as dysprosium (shown at left), and other materials, such as lead (shown at right) behave differently when a few atoms of each type of material are deposited on a graphene and the atoms self assemble into tiny islands. Rare earths appear to move slowly, suggesting strong electronic interaction, while lead moves quickly, suggesting weaker electronic interaction.
(PhysOrg.com) -- Transistors and information storage devices are getting smaller and smaller. But, to go as small as the nanoscale, scientists must understand how just a few atoms of metals behave when deposited on a surface.
Physicists at the U.S. Department of Energy’s Ames Laboratory are studying the interaction of materials that are promising for use in nanoscale electronics: graphene and different types of metals. The team has discovered the rare-earth metals dysprosium and gadolinium react strongly with graphene, while lead does not.
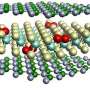
Michael C. Tringides, an Ames Laboratory senior physicist, and colleagues Myron Hupalo, an Ames Laboratory scientist, and Steven Binz, a graduate student in physics, deposited a few atoms of lead or rare-earth metals on the surface of graphene, a one-atom thick layer of carbon. In a process called self assembly, the atoms move on their own and form islands or smooth films on graphene. Tringides and the team then used scanning tunneling microscopy to study the islands’ geometry.
“We wanted to understand how the atoms diffuse, particularly how quickly,” said Tringides. “In this case, the lead atoms moved quickly when we cooled them down, while the dysprosium moved slowly, even after we heated them up.”
How fast or slow the atoms move and form islands offers insight into how each material interacts, or shares electrons, with the graphene.
“If the atoms move fast, it means you do not have strong interaction,” he said. “It’s like hockey pucks skimming along on an ice rink. There’s little interaction.”
In the case of dysprosium, the slow-moving atoms suggest that the metal reacts strongly with graphene. Gadolinium has an even stronger interaction. The interaction is significant because harnessing the potential of graphene in electronics will require attaching metals to graphene to conduct electricity.
“The hope is that graphene can be used for super-fast transistors,” said Tringides. “Our work is relevant to this because when you put metal on graphene, you want to have very good contact, so the electrical resistance is low.”
Tringides also says that the rare-earth islands on graphene are tiny magnets.
“It turned out that these islands were good nanomagnets on graphene,” said Tringides. “You have a very high density of nanomagnets. Iron also has a similar high island density. This may be useful in the future for using metals on graphene in computer memory.”
Ames Laboratory theoretical physicists C.Z. Wang and Kai-Ming Ho collaborated on the research, using calculations to confirm the experimental results about the bonds between graphene and the metals studied.
“These findings are interesting for both the fundamental physics and because of the potential usefulness,” said Tringides. “Whenever you say ‘nano,’ you can make a lot of something in a small size. And that might be very beneficial for something like magnetic computer memory.”
DOE’s Office of Science funded the research, which has been reported in the journal Advanced Materials.
Provided by Iowa State University