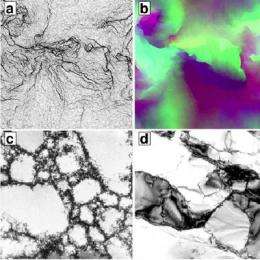
The top two images are two views of a computer-simulated fractal wall pattern after strain. The bottom two are micrographs taken from a single copper crystal and single aluminum crystal after strain.
Blacksmiths make horseshoes by heating, beating and bending iron, but what's happening to the metal's individual atoms during such a process? Cornell researchers, using computational modeling, are providing new insight into how atoms in crystals rearrange as the material is bent and shaped.
The researchers made computer-synthesized models of what such metals as aluminum and copper look like at the atomic level while being stretched, heated and cooled. They simulated how crystals, whose atoms start in a regular grid, transform as they are bent into different shapes.
Such new theories could lead to a better understanding of structural materials, from buildings to bridges, to make them less susceptible to tearing or breaking.
"We're really at the beginning stages of trying to develop a systematic theory of how materials evolve as we vary strain and temperature," said James Sethna, Cornell professor of physics, who leads the research.
The work is published in the Sept. 1 edition (Vol. 105 Issue 10) of Physical Review Letters, a publication of the American Physical Society.
When a single crystal is bent, portions of the crystal shift and create defects in the lattice called dislocations. The researchers found that their crystals exhibited starkly contrasting properties depending on temperature.
When hot crystals were bent, the dislocations arranged into grain boundaries, which are the places where lattice planes suddenly tilt. At low temperatures, the dislocations formed self-similar, random patterns known as fractals.
Provided by Cornell University