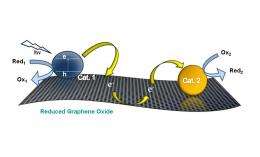
Reduced graphene oxide (RGO) can serve as a catalyst mat by anchoring particles that carry out catalysis at different sites. Image credit: Prashant V. Kamat.
(PhysOrg.com) -- Researchers have found a new use for graphene, the single-atom-thick sheet of carbon atoms that resembles chicken wire. Ever since graphene was first observed in 2004, its large surface area, excellent mechanical strength, and high electrical conductivity have intrigued scientists and opened up new areas of exploration.
In their recent study, Ian Lightcap, Thomas Kosel, and Prashant Kamat of the University of Notre Dame have demonstrated that graphene can be used as a multifunctional catalyst mat. As a catalyst mat, two-dimensional graphene can hold particles that act as catalysts to speed up or slow down the rate of chemical reactions. The findings may pave the way for the development of next-generation catalyst systems, as well as advances in chemical and biological sensors. The study is published in a recent issue of Nano Letters.
“The obvious challenge [in constructing a catalyst mat] is to have a large area of carbon surface so that the catalyst particles can be dispersed without any aggregation,” Kamat told PhysOrg.com. “Graphene, with its two-dimensional nanostructure, provides the largest surface area to anchor catalyst particles.”
In addition to its large surface area, a graphene communicating platform also has the ability to store and transfer electrons to different locations on the platform due to its redox properties. Taking advantage of these properties, the researchers used electron transfer processes to anchor two different catalyst particles - semiconductor nanoparticles (titanium dioxide) and metal nanoparticles (silver) - to the mat. As the researchers explain, having two different catalyst particles in different locations on the same sheet can provide greater versatility for carrying out catalytic processes.
To construct the catalyst system, photogenerated electrons in titanium dioxide nanoparticles are first transferred into the graphene oxide substrate. Some of these electrons are used to improve the conductivity of the substrate, turning the graphene oxide into reduced graphene oxide (RGO). Meanwhile, other electrons are stored in the RGO sheet until the introduction of silver nitrate. At this point, the stored electrons are transported across the RGO sheet to reduce the silver ions into silver nanoparticles, which serve as seeds for additional growth.
“The graphene sheet facilitates direct communication between different particles by shuttling electrons across the carbon plane,” Kamat said. “The growth of silver nanoparticles confirms the ability of the graphene sheet as an electronic communicating platform between semiconductor and metal nanoparticles anchored on the graphene sheet. … One can envisage depositing other catalyst nanoparticles to incorporate additional selectivity.”
One example that Kamat noted is a water-splitting catalyst system, in which molecular oxygen and hydrogen are generated at separate catalyst sites.
More information:
-- “Anchoring Semiconductor and Metal Nanoparticles on a Two-Dimensional Catalyst Mat. Storing and Shuttling Electrons with Reduced Graphene Oxide.” Ian V. Lightcap, Thomas H. Kosel, and Prashant V. Kamat. Nano Letters. DOI:10.1021/nl9035109
-- “Graphene-Based Nanoarchitectures. Anchoring Semiconductor and Metal Nanoparticles on a Two-Dimensional Carbon Support.” PraDOI:10.1021/jz900265je Journal of Physical Chemistry Letters. DOI:10.1021/jz900265j
Copyright 2010 PhysOrg.com.
All rights reserved. This material may not be published, broadcast, rewritten or redistributed in whole or part without the express written permission of PhysOrg.com.