(PhysOrg.com) -- Bacteria use all sorts of cunning to trick hosts into doing their bidding. One con in their bag of tricks: the molecular mimic. In this ruse, bacteria or their agents look for all purposes like some native molecule in a cell, but then do not behave accordingly. Working with H. pylori, the bacterium responsible for gastric ulcers and cancer, researchers have revealed one way bacteria pull this off, deciphering the structure of a piece of CagA, a bacterial protein that impersonates a human protein in order to disable a key enzyme.
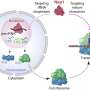
Helicobacter pylori infects up to 90 percent of people in the developing world and causes gastric ulcers and cancers of the gut. Now scientists have revealed a subterfuge used by the bacterium to trick stomach cells into playing along. By injecting a protein into the stomach lining that mimics a native protein but has its opposite effect, the bacterium shuts down a process that helps properly structure stomach tissue, scientists say.
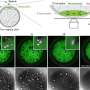
C. Erec Stebbins, head of the Laboratory of Structural Microbiology at Rockefeller University, Research Associate Dragana Nesic and colleagues deciphered the atomic structure of an important segment of the large H. pylori protein CagA as it attached to a human enzyme called MARK2. MARK2 (also known as PAR1b) regulates processes including the “tight junctions” that form between cells, packing stomach tissue together. Using the technique of x-ray crystallography, the researchers captured CagA bound to MARK2 and established the position of each atom surrounding the interaction by interpreting the pattern of x-rays diffracting from a crystallized structure of the union.
The team, including biomedical fellow Marshall Miller and Brian T. Chait’s Laboratory of Mass Spectrometry and Gaseous Ion Chemistry, published the experiments December 6 in Nature Structural & Molecular Biology. “It was the first time anyone has ever imaged CagA interacting with a human protein,” Stebbins says. “We know CagA basically shuts down MARK2, disrupting different cell functions, and we wanted to find out how that happens. We start with structure and move on to function.”
The researchers performed a series of biochemical tests, creating CagA mutants missing individual amino acid residues to determine which ones are crucial to its interaction with MARK2. Nesic identified four key elements in a “binding pocket” of the protein in an arrangement that is strikingly similar to many of the body’s native proteins that interact with MARK2. “Evolution has created a bacterial protein — CagA — that looks exactly like one of ours, and the enzyme that interacts with it is totally fooled,” Stebbins says. “CagA binds to it so tightly that the enzyme gets locked in this trapped, dead state and is unable to do what it usually would.”
The work by Nesic and Stebbins looks at only one part of the large protein, but their success in detailing the structure and function of this important element shows that it’s possible to dissect the protein one piece at a time. H. pylori is known for its direct involvement in gastric ulcers and tumors, and the activity of the enzyme that CagA effectively shuts down has been implicated in other disorders, including Alzheimer’s disease and obesity. So understanding more about how CagA works is potentially useful for treating a litany of medical problems.
“What we hope is that now we’ve opened up CagA by showing how we can take this huge protein on,” Stebbins says. “We would love to see this kind of research accelerate because there is a lot more we need to understand about how it works.”
More information: Nature Structural & Molecular Biology online: December 6, 2009; Helicobacter pylori CagA inhibits PAR1-MARK family kinases by mimicking host substrates; Dragana Nesic, Marshall C. Miller, Zachary T. Quinkert, Markus Stein, Brian T. Chait and C. Erec Stebbins
Provided by Rockefeller University (news : web)