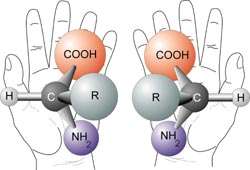
Most types of amino acids exist in 'left-handed' and 'right-handed' forms. Compounds with this property are known as chiral, and exist as mirror images of each other. Life as we know it makes and uses only 'left-handed' amino acids. Credit: NASA
Molecules vital to life have been detected in outer space and isolated in meteorites and comets. Some of this material that rained down on Earth may have jump-started biology. If so, these space seeds also may have planted a particular molecular orientation, or "handedness," that spread to the world's first creatures. New research is studying how this handedness could arise in space.
Amino acids, the building blocks of proteins, exist in two so-called “chiral” forms that are mirror reflections of each other, like a left and right hand. For some unknown reason, organisms use left-handed amino acids almost exclusively in making proteins (the other mirror image, while rare, is sometimes used in other processes).
"Outside of biology the ratio of these chiral forms is 50-50, so we want to understand the starting point of life's preference for left-handed amino acids," says Orlando Santos of NASA Ames Research Center.
Santos and his colleagues are designing a small satellite that would carry up biologically relevant molecules to see what effects space has on a sample's handedness, and whether this could explain the origins of homochirality.
"Other researchers in this field have tried to reproduce space conditions in a lab," Santos says. "But artificial systems are just that. We want to test the theories in a natural environment."
The project is part of the Astrobiology Science and Technology Instrument Development and Mission Concept Studies. In a follow-up story, Astrobiology Magazine will profile another NASA-supported experiment that hopes to address how handedness in space might be delivered to the ground.
Meteor impact
The evidence for a space origin for homochirality comes from meteorites. Several of these space rocks contain amino acids, and in a few cases the left-handed amino acids have outnumbered the right-handed ones by as much as 15 percent.
"There is no doubt about the left-handed excess in meteorites," says Sandra Pizzarello of Arizona State University, who has done extensive analysis of various meteorite samples. She has found that they contain higher than normal levels of the isotopes deuterium and carbon-13, which would argue that the molecules came from space and are not simply contaminants from Earth.
"The isotope ratios imply that at least parts of each molecule formed in a cold space environment at less than 50 degrees above absolute zero," says George Cooper of NASA Ames.
Space factors
However, it is unknown what mechanism in space could impart handedness to amino acids or any other molecules.
The most popular candidate is circularly polarized ultraviolet light. Amino acids are broken apart by ultraviolet light, but if this light is circularly polarized (rotating in a clockwise or counterclockwise direction), the destruction of one hand will be faster than the other.
"Both hands decompose in the light, so it turns into a race toward total destruction," Pizzarello explains. "An excess of one hand can be made this way, but there will only be a small percentage of the original material left over."
The maximum excess that has been generated in the lab has been 9 percent, according to Pizzarello. This is considerably less than what is observed in the meteorites, so it's unclear what would make up the difference.
A computer drawing of the planned O/OREOS nano-satellite, on which the EOOSS design is based. Credit: NASA Ames
Another concern is that circularly polarized light has never been detected in ultraviolet wavelengths coming from an astronomical source. It has only been inferred to exist around neutron stars and inside dense molecular clouds where stars are forming.
An alternative possibility is that magnetic fields can help to promote one chiral form over the other. Louis Pasteur, who was the first to observe homochirality in a biological sample, imagined that magnetic fields might play an important part.
Later theoretical work showed that a magnetic field lined up with ultraviolet light can destroy one chiral form more readily that the other, just as in the case of circularly polarized light.
"In our solar system, you are more likely to encounter a magnetic field than circularly polarized light," Cooper says.
However, many scientists have tried but failed to create higher concentrations of one chiral form with magnetic fields. It wasn't until 1997 that anyone succeeded in showing the magnetic effect, but it took a huge field of more than a Tesla, which is 10,000 times the Earth's magnetic field.
A go-and-see policy
With no clear mechanism at play, it may be that several factors in space work together to produce an excess of one hand over the other.
"The meteorites were exposed to a variety of effects - some that we don't even know yet," Cooper says.
Cooper is working with Santos, Arthur Weber and others in the NASA Ames Exobiology Branch to develop a simple satellite based on the design of another experiment called O/OREOS. This new satellite - entitled Exposure of Organics On a Small Satellite (EOOSS) - will be used specifically study chiral effects in space. The plan is to carry up amino acids and other organic compounds and expose them to UV light from the sun and the magnetic field from the Earth.
"The light and magnetic field can be produced in a lab, but we think other factors could be important like microgravity," Santos says. "The lack of gravity might help orient molecules, so you could get away with a smaller magnetic field."
An on-board polarimeter will record the polarity of light going through the samples, and thereby determine if one hand of molecules begins to dominate the other. Data will be collected in real-time and beamed down to Earth.
The Earth's magnetic field probably had little influence on organic compounds falling onto the planet from space. However, it can serve as a proxy for magnetic fields that presumably existed billions of years ago when these compounds formed.
"We want to simulate the early solar system environment of these organics as best we can," says Cooper.
Devil in the details
"It's always good to go and test something in a real setting," says Pizzarello, who is not involved with the project.
However, she is not sure that the Earth's magnetic field will be strong enough. "The devil's in the details," she says.
Right now, Santos and his colleagues are working on their design, and they expect in January 2010 to have a final decision on whether to go forward and build their satellite. If all goes well, a launch could happen in 2011-12, Santos says.
Source: Astrobio.net, by Michael Schirber