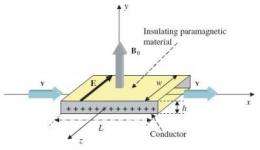
In this illustration of the system, salt water flows through a rectangular pipe under the influence of a perpendicular magnetic field, B0. The Lorentz force causes the charged sodium and chlorine ions to accumulate near the metal plates on the sides of the pipe, generating a constant electric field, E. Image credit: R. De Luca.
(PhysOrg.com) -- The idea of generating hydrogen from salt water has often been claimed to work effectively. However, the systems proposed so far generally require a much greater energy input than the energy they produce, making them impractical for energy generation. Now, a recently revived system may be able to cheaply generate a small amount of power.
In the proposal, physicist Roberto De Luca from the University of Salerno in Italy has suggested that flowing salt water could generate an electromotive force, which in turn could generate an electric power output. In his theoretical analysis, he considers letting salt water (containing sodium and chlorine ions) run through a rectangular pipe that has two metal electrodes on the sides, under the influence of a perpendicular magnetic field. In this set-up, the Lorentz force acts on the sodium and chlorine ions in the salt water, creating a Faraday voltage across the two electrodes, and producing an electromotive force.
"I started considering the question Dr. Pasquale Desideri, a Roman chemist, asked," De Luca told PhysOrg.com. "If a transverse magnetic field is applied to salt water flowing in a thin rectangular pipe, would an electromotive force appear at the sides of the pipe itself? This question was interesting both from a didactical and a scientific point of view. Didactically, one could come up with an interdisciplinary lecture, making a comparison between the electrical transport properties of the 'Fermi sea' (the collection of free electrons in a metal) and the 'ordinary sea' (which a physicist could conceive as a collection of rather free Na+ and Cl- ionic charges diluted in water, besides a place to go in summertime)."
De Luca discovered that an experiment conducted in 1972 (by Wright and Van Der Beken) had demonstrated that Desideri's hypothesis was true: salt water flowing in a pipe under a transverse magnetic field does show an effect, similar to the Hall effect, in conducting metals. De Luca thought that this simple fact deserved more attention.
As he showed in his analysis, in order to produce a steady current in this device, the positive and negative electrodes experience different reactions. At one electrode, water is reduced to its components, resulting in oxygen and hydrogen gas. At the other electrode, chlorine ions are oxidized, producing chlorine gas.
De Luca investigated the minimum concentration of sodium chloride in the water required to maintain a steady current. He calculated that only a small concentration of the ions is needed to establish a potential difference between the electrodes, and normal salt water has a significantly higher concentration than required. (The average salinity of seawater is about 3.5%, and about 78% of these salts is sodium chloride.)
The technique also requires that the salt water flow through rectangular pipes with a very small height (so that they are nearly one-dimensional). Although the technique wouldn't work in most natural locations, De Luca suggests that some desalination plants - where salt water is forced to run through small ducts - may provide an adequate infrastructure for the system. If so, desalination plants might also function as alternative electric power sources.
"When I was sure that an electromotive force and hydrogen gas could be obtained from letting salt water circulate in the presence of a transverse magnetic field, I thought about the huge desalination plants, where intake of salt water from the sea is needed. In general, these plants are run by oil, even though they are mostly located in places where solar power is present in abundance. Getting some power out of these plants simply by applying a transverse magnetic field to pipes could mean saving some power. Besides, getting hydrogen gas from sea water could gives us hope that in the future we would not easily run out of fuel.'"
There are still some challenges that this scheme faces, but De Luca has showed the potential using this method to produce hydrogen gas in a natural and inexpensive way. Apart from applications, the concept could be used in introductory physics classes to teach students about the transport properties of ionic aqueous solutions and conducting materials.
"I am now sure that most people will come up saying that these applications are rather difficult to implement and, even though some uses of this simple theoretical analysis can be envisioned, the amount of power one can derive from these systems is rather small," he said. "Scientifically, however, one is mainly concerned with the clear statement of some well-defined facts. In particular, I can say that it can be nowadays stated that hydrogen gas can be cheaply produced by solar energy, as already noticed in a previous paper, and can also be produced by simple electrodynamic effects. How cheaply in the latter case? Consider that seas do not ever stand still and that, luckily enough, there exist so called permanent magnets."
More information: De Luca, R. "Lorentz force on sodium and chlorine ions in a salt water solution flow under a transverse magnetic field." European Journal of Physics, 30 (2009) 459-466.
© 2009 PhysOrg.com