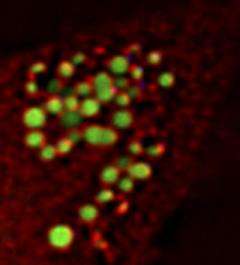
Spartin (red) localizes on the surface of lipid droplets (green). Credit: Eastman, S.W., et al. 2009. J. Cell Biol. doi:10.1083/jcb.200808041
Spartin, a protein linked to the neuronal disease Troyer syndrome, was thought to function in endocytosis. In the March 23, 2009 issue of the Journal of Cell Biology, Eastman et al. identify an unexpected role for Spartin in regulating the cell's lipid storage depots.
Cells transport excess fats into lipid droplets (LDs), where they are kept until needed as sources of energy, but little is known about LD formation and regulation. Spartin hadn't been connected to LDs. But Eastman et al. found that Spartin localizes to LDs and binds to a known LD protein called TIP47.
Spartin seems to regulate LD turnover: overexpressing or knocking-down the protein caused an increase in both the organelle's size and number. In turn, Spartin is regulated by ubiquitination by the ubiquitin ligase WWP1. Overexpressed WWP1 removed Spartin from LDs and promoted its degradation.
A truncated form of Spartin found in a rare neuronal disease called Troyer syndrome didn't localize to LDs or bind to TIP47. It remains to be seen whether defects in LD function are the root cause of the defects seen in Troyer patients, but author Paul Bieniasz points to precedents of defects in lipid turnover triggering neuronal disorders, such as mutations in the phospholipase NTE causing motor neuron disease.
More information: Eastman, S.W., et al. 2009. J. Cell Biol. doi:10.1083/jcb.200808041. (www.jcb.org)
Source: Rockefeller University (news : web)