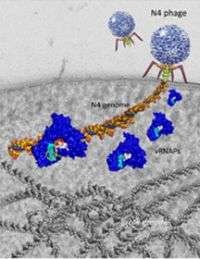
The researchers uncovered clues that may explain how and why a particular virus, called N4, injects an unusual substance -- an RNA polymerase protein -- into an E. coli bacterial cell. In this cryo-electron micrograph image, an N4 phage virus is shown sitting on the surface of a bacterial cell. It is injecting a molecule of DNA (shown in red, yellow, and blue) and four RNA polymerase proteins (shown in blue) into the bacterial cell. In addition, the bacterial cell's DNA (shown in gray) is visible at the bottom of the image. Credit: K.H. Choi and J.D. Hayden, Penn State
(PhysOrg.com) -- A team of researchers from Penn State University and the University of Chicago has uncovered clues that may explain how and why a particular virus, called N4, injects an unusual substance -- an RNA polymerase protein -- into an E. coli bacterial cell. The results, which are published in the current issue of the journal Molecular Cell, contribute to improved understanding of the infection strategies used by viruses that attack bacterial cells. Such viruses are known as bacteriophages, or phages. The results also may help other researchers to come up with new ideas about ways to kill E. coli bacteria, which can be dangerous to humans.
"Most phages inject only their own DNA into bacterial cells," said Katsu Murakami, a Penn State assistant professor in the Department of Biochemistry and Molecular Biology and a leader of the study. "These phages then use the host bacterial cell's RNA polymerase to synthesize messenger RNA through a process called transcription, which ultimately results in the creation of new phage proteins. These new proteins are used to construct new phages inside the bacterial cell. But the phage that we are studying is different. It injects both its own DNA and its own RNA polymerase into bacterial cells, so it can begin the process of transcription without any help from the bacterial host's RNA polymerase."
The team says that the N4 phage that they are studying is the only phage that they know of that injects its own RNA polymerases into bacterial cells. "We are particularly interested in finding out why N4 injects its own RNA polymerase into bacterial cells and how the N4 RNA polymerase finds the N4 DNA and initiates transcription -- and, ultimately, the creation of new N4 phages -- once it is inside a bacterial cell," said Murakami.
To begin to answer these questions, team member Michael Gleghorn, a former graduate student in the Penn State Department of Biochemistry and Molecular Biology who is now a postdoctoral researcher at the University of Rochester, used X-ray crystallography to obtain a high-resolution three-dimensional image of the N4 phage's RNA-polymerase and DNA molecule. "By modifying the crystallography conditions, Michael obtained an extremely high-resolution picture of the N4 RNA polymerase and DNA molecule. So we are able to analyze protein-DNA interactions much more clearly," said Murakami.
The picture of this RNA polymerase and DNA molecule has enabled the team to investigate how the RNA polymerase initiates transcription of phage DNA from inside a bacterial cell. "When a phage injects its DNA into a bacterial cell, the amount of its DNA is miniscule compared to the amount of host DNA," said Murakami. "We wanted to find out what prevents the N4 RNA polymerase from binding to the bacterial host's DNA rather than to the phage's DNA."
It turns out that the N4 RNA polymerase is able to respond only to DNA that is shaped like a hairpin. Part of the N4 phage's DNA is shaped like a hairpin, whereas the E. coli bacterium's DNA is not shaped like a hairpin. Once the N4 RNA polymerase interacts with the phage's hairpin DNA, it begins to change its shape from a fisted form to a cupped form. By opening up, the RNA polymerase exposes its active site, which allows it to begin the transcription process.
While the researchers determined that the N4 RNA polymerase must change its form in order to bind to the phage DNA, they also found that this transformation isn't the polymerase's first as it progresses through the steps of phage infection. The team found that the polymerase must change form in order to squeeze through the phage's tiny injection tube as it is injected into the E. coli cell. "The diameter of the tube is narrower than the diameter of RNA polymerase," said Murakami. "This means that the enzyme must be unfolded into a longer and thinner structure in order to fit through the tube, and then it is refolded after it is injected into the cell."
The ability of the N4 RNA polymerase to withstand this unfolding and refolding is unique. Therefore, the team decided to experiment with this property by exposing the polymerase to high temperatures. As expected, the high temperatures caused the molecule to unfold. The scientists then cooled the molecule and watched as it reformed into its original shape and regained its functions.
In addition to helping scientists to advance their understanding of the process by which phages infect bacterial cells, Murakami hopes that the novel infection strategy of the N4 phage will be useful in the development of new therapeutic methods for killing E. coli. "The N4 virus injects its own RNA polymerase, which is a type of protein, into the E. coli cell. This system could be replicated and used to deliver proteins or drugs that kill the bacterium," said Murakami. This research was supported by the National Institutes of Health.
Source: Penn State