November 28, 2008 feature
Gas pump made of minerals has no moving parts
(PhysOrg.com) -- Scientists have discovered that a type of hard mineral called zeolite can provide a high rate of gas flow in a micro-scale gas pump. Because the pump is based simply on temperature differences and has no moving parts, it could provide reliable and precise control of gas flow for a variety of applications, such as gas-sensing breath analyzers and warfare agent detectors.
Mechanical engineers Naveen Gupta and Yogesh Gianchandani from the University of Michigan have published their study on the zeolite gas pump in a recent issue of Applied Physics Letters. The researchers used a type of zeolite called clinoptilolite that, like all zeolites, contains billions of nanopores which a gas can flow through. The nanopores in clinoptilolite are packed much more densely than could be achieved through lithographic techniques, and so the mineral can enable a higher rate of gas flow.
“Unlike zeolite gas pumps, most of the traditional micropumping mechanisms have moving parts,” Gupta told PhysOrg.com. “As we go smaller in size, the ratio of the surface area to volume of the parts increases, which results in increased frictional losses of power. Larger frictional forces result in increased wear and tear in the devices, which affects the reliability of the system adversely.”
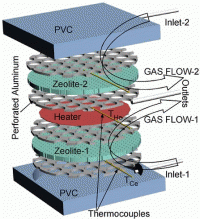
Using clinoptilolite, the engineers built a gas pump that operates on the principle of thermal transpiration, the phenomenon that gas molecules drift from the cold end to the hot end of a narrow channel. Their handheld-size gas pump, called a Knudsen pump, consisted of a thin, flexible heater in the center, sandwiched between two thin pieces of the porous mineral. The researchers added pieces of perforated aluminum between the layers to maintain a uniform temperature. Finally, the entire assembly was sandwiched between two pieces of insulating polyvinyl chloride (PVC).
The pump operated on 296 mW/cm2 of power, and contained two inlet ports for the gas to enter at opposite ends of the device. When the heater started operating, cold gas molecules from the inlet ports began drifting through nanopores in the zeolite mineral toward the heater in the center. The gas quickly flowed out through a central outlet port, and could be used for a specific application.
As the researchers explained, the thinner the nanopores (or nanochannels) through which the gas flowed, the higher the pressure at which the pump could operate. Using the zeolite’s large number of thin nanochannels, the device could pump gas at a high flow level. These nanochannels were so thin (in this case, about half a nanometer), that they were thinner than the mean free path of the molecules at atmospheric pressure, resulting in “free molecular gas flow.”
“The free molecular regime is a name given to the gas flow conditions in which the mean free path of the gas molecules is much larger than the characteristic length of the channel,” Gupta explained. “Unlike the case for the continuum gas flow regime, in the free molecular regime the gas molecules bounce against the channel walls much more frequently than they bounce against each other. Under these conditions, the wall interaction dominates and tends to cause the molecules to drift from the cold end to the warm end of the channel.”
Clinoptilolite, which has a greenish-white color, is one of the most abundant zeolites, and is also inexpensive, easily accessible, and mechanically strong. Along with having no moving parts, these advantages may make the pump useful for various purposes.
“These miniature pumps may someday be useful for a variety of applications ranging from ventilation to vacuum pumping,” Gupta said. “They may also assist as gas reservoirs and gas separation elements in miniature or handheld system diagnostic. However, the Knudsen pumping technology is still evolving and will need quite a bit of effort before it gets there.”
More information: Gupta, Naveen K. and Yogesh B. Gianchandani. “Thermal transpiration in zeolites: A mechanism for motionless gas pumps.” Applied Physics Letters 93, 193511 (2008).
Copyright 2008 PhysOrg.com.
All rights reserved. This material may not be published, broadcast, rewritten or redistributed in whole or part without the express written permission of PhysOrg.com.