Getting to grips with the complexity of disease proteins
Drug molecules seldom act simply on one protein but on protein complexes and networks. A deeper understanding of these 'cooperative assemblies' should lead to better targeting of drugs
New research into how proteins in human cells interact and 'talk' to each other is leading to a better understanding of how drug molecules work and should result in more effective therapies, according to a leading European scientist.
"Most of the time the mechanism of action of drugs is ill understood and we often do not even know the primary target of the drugs we swallow daily," Professor Giulio Superti-Furga of the Centre for Molecular Medicine of the Austrian Academy of Sciences said. "We do not know how these drugs work at the molecular level, and side effects can have serious consequences."
Superti-Furga was speaking at the European Science Foundation's 3rd Functional Genomics Conference in Innsbruck, Austria, held on 1-4 October. Functional genomics describes the way in which genes and their products, proteins, interact together in complex networks in living cells. If these interactions are abnormal, diseases can result. The Innsbruck meeting brought together more than 450 scientists from across Europe to discuss recent advances in the role of functional genomics in disease.
Our lack of understanding of the way that drugs work is illustrated by the fact that around four in ten drugs currently on the market were developed for one use but were subsequently found to be better for a different condition.
Researchers such as Superti-Furga are taking a taking a 'proteomics' approach to understanding precisely how certain proteins that are key drug targets organise themselves in the cell, and how they make complex interactions with often dozens of other proteins. "Proteomics is a way of joining the dots together to give us the bigger picture," he said.
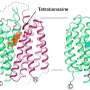
Superti-Furga's team has been investigating a particular enzyme, a tyrosine kinase called Bcr-Abl, which is involved in leukaemia. A drug is available that acts on the enzyme, but it eventually loses its efficiency as patients become resistant to it. "We need to understand the relationship between the drug and the target," said Superti-Furga. "Can we understand the 3-d protein as a molecular machine much better?"
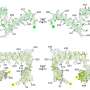
Superti-Furga's lab in Vienna has used a range of proteomics techniques to isolate the enzyme and dissect its constituent parts. They discovered that the protein exists as a complex of some 46 separate components and operates as a giant molecular machine, with each part in close communication with the others.
"It is clear that tyrosine kinase inhibitors do not simply inhibit the enzyme, but rather remodel the machine," Superti-Furga aid. "So drugs do not simply ablate things, they interfere with the equilibria of networks. If we can understand how these proteins interact, in the future people might say we should target this pathway or that network'; by targeting multiple nodes we will be able to maximise the good side effects against the bad side effects."
The team has also been developing methods to understand how the human body can recognise invading foreign genetic material – comprising nucleic acids – from bacteria or viruses for example, and distinguish it from its own, innate genetic material.
It is thought that proteins in the human cell can tell if a sequence of nucleic acids is from an invading organism. To try to identify these proteins the research team has developed a technique to test which proteins in a cell bind specifically to foreign nucleic acids. They have also observed which genes in the cell are switched on or 'up regulated' when foreign genetic material is present – and whether the proteins that are encoded by these genes are the same as those that bind to the material. A number of candidate proteins have emerged from this process and are undergoing further study.
The work will provide important insights into how the body defends itself against invading organisms.
Source: European Science Foundation