Scientists produce carbon nanotubes using commercially available polymeric resins
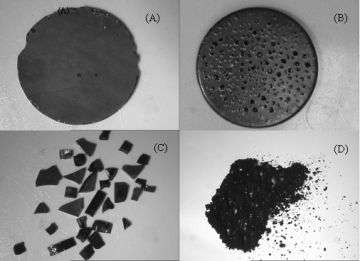
Scientists at the Naval Research Laboratory (NRL) have successfully produced carbon nanotubes (CNTs) in high yields in bulk solid compositions using commercially available aromatic containing resins. The concentration of multi-walled carbon nanotubes (MWNTs) and metal nanoparticles can be easily varied within the shaped carbonaceous solid.
Carbon nanotube containing fibers and films have also been formulated from the precursor compositions. The potential range of applications is huge, including structure, energy, sensors, separation/filtration, battery, electronic displays, and nanoelectronic devices.
Using this method, carbon nanotubes (CNTs) are formed in a bulk carbonaceous solid from thermal decomposition of melt-processable precursor compositions formulated from organometallic compounds or metal salts in the presence of an excess amount of selected highly aromatic compounds.
The CNTs obtained by this patented method are not formed from gaseous components, as is common with the current CNT production based on chemical vapor deposition (CVD) methods, but rather evolve from metal and carbon nanoparticles that form within the carbonaceous solid during the carbonization process above 500°C. Only a small amount of the organometallic compound or metal salt is needed to achieve the formation of CNTs in high yield, but large quantities of the metal source can be used, depending on the application, if desired.
The solid-state method enables the large-scale production of MWNTs in moldable solid forms, films, and fibers using low-cost precursors and equipment, thereby reducing economic barriers that are inherent with carbon nanotube materials produced by more conventional methods, such as CVD.
Following carbonization, the shaped carbon solids are composed of varying amounts of nanotubes and amorphous carbon, depending on such synthetic parameters as the metal catalyst concentration, carbonization temperature, and the specific organic precursors used. The amorphous carbon phase is readily removed via selective combustion at temperatures from 300–500 °C, producing highly porous, purified CNT solids with specific surface areas up to 500 m2 g-1. This highly flexible synthetic method also offers the ability to incorporate heteroatoms, for example nitrogen, oxygen, and/or boron, into the carbon nanotube solid via the initial carbon precursors.
The NRL scientists use standard resin melt processing techniques to produce various shaped CNT-containing carbonaceous configurations. Their research is the first example of using high temperature thermosetting resins as a carbon source for the formation of CNTs. Any commercially available resins, including phthalonitriles resins, polyimides, epoxy resins, phenolics, and petroleum pitches, that have good thermal properties and show superior structural integrity, are attractive sources of carbon for CNT formation by the novel method.
The use of commercially available resins is a potentially inexpensive route to CNTs. Using this simple, potentially cost-effective method could result in the production of CNTs in large quantities and various shapes. Scientists are evaluating them for possible use in numerous aerospace, marine, and electronic applications.
Source: Naval Research Laboratory





















