October 30, 2006 feature
Ancient Hair-Dyeing – A Nanoscience?
Scientists have discovered that an ancient method used to darken hair, dating back more than 4,000 years, is based on a chemical process that takes place at the nanoscale. This may be one of the earliest examples of nanoscience at work in a practical application.
The research team is led by Dr. Philippe Walter, a chemist with the Centre Nationale de Recherche Scientifique (National Center for Scientific Research) in Paris, France. For the past 10 years, he and his group have collaborated with the research department at L'Oreal, studying the history of cosmetic science.
This study was inspired by one of their early projects, an investigation of ancient Egyptian cosmetics in which they analyzed samples preserved at the Louvre Museum. They demonstrated that the Egyptians used wet chemistry to synthesize lead chloride compounds, which were added to the black pigment lead sulfide (PbS), also known as galena, to confer medicinal properties to eye makeup, in order to treat eye illnesses. This practice is described in Roman writings dating back to the first century A.D.
“Since that study, we have developed new research on materials from the Greek and Roman periods. When I found an ancient recipe dealing with the use of lead to dye hair black, I considered it another way to understand how ancient cultures developed lead-based chemistry for health and beauty needs,” Walter told PhysOrg.com. “We reconstituted the recipe and observed the formation of galena nanocrystals in the hair.”
The use of lead compounds to create dyes was common during the Greco-Roman period, and similar formulas were still in use even up to modern times. A particular recipe that has been recorded in several places over the centuries consists of a mixture of lead oxide (PbO) and calcium hydroxide (Ca(OH)2), with a little water added to form a paste that is then applied to the hair. Scientists have known that the blackening of the hair is due to the precipitation of PbS crystals during the treatment, in which the sulfur comes from a reaction with keratins (the amino acids present in hair proteins). However, this is the first time that scientists have uncovered the finer details of the process.
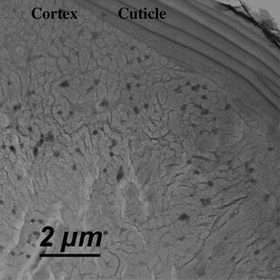
In a paper describing the work, published in the September 1, 2006, edition of Nano Letters, Walter and his colleagues report the shape and distribution of the nanocrystals within the hair, which they gathered by detecting the compound's lead ions using x-ray analysis and electron microscopy. The PbS nanocrystals are about five nanometers in size on average, but clump together in globular 200 nm aggregates. They collect mainly in the hair's cortex (the middle layer), and tend to arrange themselves in lines along the axis of the hair. These lines are about 8-10 nm apart, which roughly matches the distance between long, thin keratin fibers, or “microfibrils,” in the cortex. Thus, it appears that the alignment of the galena nanocrystals is induced by the organization of the microfibrils.
What is particularly surprising about the reaction, Walter remarks, is that the galena particles easily crystallize and organize within the hair, a biomaterial, despite the structural complexity of hair and its relative inertness. He adds that the nanocrystals are quite similar in appearance to PbS “quantum dots” synthesized using modern materials science techniques. “But unlike modern nanotechnology, this dyeing process is characterized by basic chemistry methods and was developed thousands of years ago with low-cost, natural materials,” he says.
Currently, he and his group are studying how other metal ions crystallize and diffuse within hair and are assessing the feasibility of using the hair structure – its “matrix” – as a nanoreactor. Being able to better control the growth and organization of nanoparticles in an organic material could lead to new advances in the development of nanomaterials.
By Laura Mgrdichian, Copyright 2006 PhysOrg.com