Renewable Raw Materials
Petroleum and natural gas reserves are getting smaller and smaller. It is thus a real waste to burn up these valuable resources for heat or transportation especially as "black gold" is also the most important starting material for the chemical industry.
It is used in the production of most organic compounds, be they plastics, medicines, or solvents. We clearly need alternatives and are scouring nature in the hope that renewable plant resources will eventually provide some real competition for fossil resources.
For example, the enzymatic extraction of cellulose from wood by-products produces the sugar glucose, which is then fermented to form ethanol. This ethanol can then be used as a “biological” fuel for vehicles. Under different reaction conditions, the fermentation of glucose produces glycerol. Glycerol is also a highly promising starting material for the synthesis of fuels and other organic compounds, as a team of scientists from the USA and Brazil have discovered.
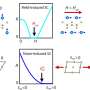
J. A. Dumesic and co-workers have developed a process in which platinum-based catalysts are used to break down glycerol to hydrogen and carbon monoxide under relatively mild conditions—temperatures between 225 and 300 °C. This process has several advantages. Among them, glycerol is currently a low-value by-product in the production of biodiesel, and fermentation of glucose produces a 25% solution of glycerol, while the degradation of sugar to ethanol results in a mixture that contains only 5 % of the desired substance. The ethanol must then be separated from this mixture by an energetically demanding distillation, whereas the glycerol-containing solution can be used as is either to produce methanol or to generate longer-chain alkanes in the Fischer–Tropsch process.
In the Fischer–Tropsch synthesis, a 2:1 mixture of hydrogen and carbon monoxide is passed over a cobalt catalyst and heated to 200 °C. Careful selection of the Pt catalyst system allows the ratio of the gases produced in the degradation of glycerol to be adjusted to the value suitable for the Fischer–Tropsch process.
The energy balance for these coupled reactions is also quite favorable: the endothermic degradation of glycerol requires an energy expenditure of 350 kJmol-1. The Fischer–Tropsch synthesis is, in contrast, an exothermic reaction, delivering -412 kJmol-1 of energy. There is thus an overall gain in energy of -62 kJmol-1.
Reference: James A. Dumesic, Glycerol as a Source for Fuels and Chemicals by Low-Temperature Catalytic Processing, Angewandte Chemie International Edition 2006, 45, No. 24, 3982–3985, doi: 10.1002/anie.200600212
Source: Angewandte Chemie