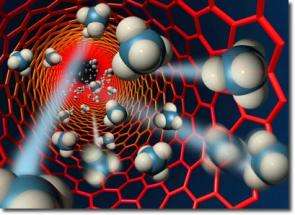
Artist’s rendering of methane molecules flowing through a carbon nanotube less than two nanometers in diameter. (Scott Dougherty, LLNL)
Surprisingly, gas and water flow far more rapidly through membranes that use carbon nanotubes as pores than through conventional membranes with pores 10 times or so wider, experts tell UPI's Nano World.
"This is like having a garden hose that can deliver as much water in the same amount of time as a fire hose that is 10 times larger," said researcher Olgica Bakajin, a physicist at Lawrence Livermore National Laboratory in Livermore, Calif.
These findings could lead to more efficient filters. "The two biggest applications we see are the separation of industrial gases and water purification, demineralization and desalination," said Jason Holt, a materials scientist postdoctoral researcher also at Livermore.
This research could also enhance fundamental knowledge of how mass flows at tiny scales. This could impact design of the microfluidic chips under development for genetic analysis or what scientists know of how the pores through which ions and water flow into cells work.
Double-walled carbon nanotubes are each roughly six water molecules wide. Classical models of gas and water flow would predict the nanotube membranes should have similar gas permeabilities to conventional polycarbonate membranes and water permeabilities 10 percent that of polycarbonate, even taking into account the differences in the number and size of the pores in question. The nanotube membranes have three or four times more area dominated by the open space of pores than polycarbonate membranes do.
What the researchers surprisingly found in membranes made with the nanotubes were "gas permeabilities that are 1,000 percent and water permeabilities that are 10,000 percent greater than for polycarbonate," Bakajin said. She and her colleagues reported their findings in the May 19 issue of the journal Science.
The Livermore and University of California at Berkeley team attributes its findings to the breakdown of classical models at the infinitesimal length scale of carbon nanotubes. Classical models do not account "for the 'billiard ball-like' collisions between gas molecules and the nanotube surface, which would enhance the gas transport rates," she explained.
Classical models also do not account for the intrinsic slipperiness of nanotube surfaces that possess virtually atomic smoothness, which may help account for the water-flow enhancement. In addition, molecular ordering processes may occur at the tiny one to two nanometer length scales of single-walled carbon nanotubes. The molecules may interact with each other to orient themselves in a chain along the axis of the nanotube. They then may move together. "The motion is rapid because they all move together," Bakajin said.
"The most exciting moment was when we saw water coming out of the membranes," Bakajin recalled. She explained the outside surfaces of carbon nanotubes are extremely water repellant, so while computer simulations indicated water would enter the nanotubes, "we were very skeptical."
"The first time we set up an experiment with water, we left it overnight thinking that the water level above the membrane would not budge," said researcher Hyung Gyu Park, a University of California at Berkeley mechanical-engineering graduate student and student employee at Livermore. "Instead, we came back in the morning and there was a little puddle on the floor under the membrane."
A criticism the research might draw is that the fast flows seen were not due to special properties of nanotubes but rather because of cracks and gaps in the membrane. "The membrane wasn't broken. We could filter out two-nanometer gold with it but the water went through fast," Bakajin said.
Chemical engineer David Sholl at Carnegie Mellon University in Pittsburgh suggested future research should focus on whether carbon nanotube membranes can show high selectivity in what they can filter, as well as begin the challenging scale up to mass production.
"Of fundamental interest will be studies that may uncover whether fluid flow through metallic versus semiconducting carbon nanotubes differs, or is diameter alone all that matters?" said physical chemist Rod Ruoff at Northwestern University in Evanston, Ill. "Coating the inner surfaces of nanotubes with any number or kind of chemical groups to cause them to impede the flow of some molecules more than others is of interest for separation technologies. Also, flow through other kinds of nanotubes, such as ones made of boron nitride, can be explored as well, as their behavior might differ from that of the carbon nanotubes."
Computational nanoscientist Jeff Grossman at the University of California at Berkeley added future research could explore to what extents the various factors Bakajin and her colleagues suspect could lead to such fast flows actually contribute to this rapidity. "Water, for all of its importance, lying at the heart of life as it does, is still something we have a lot to learn about," he said.
Copyright 2006 by United Press International