A first: Hydrogen atoms manipulated below surface of palladium crystal
For the first time, scientists have manipulated hydrogen atoms into stable sites beneath the surface of a palladium crystal, creating a structure predicted to be important in metal catalysts, in hydrogen storage and in fuel cells. The research will be published in the Dec. 13 issue of the journal Proceedings of the National Academy of Science.
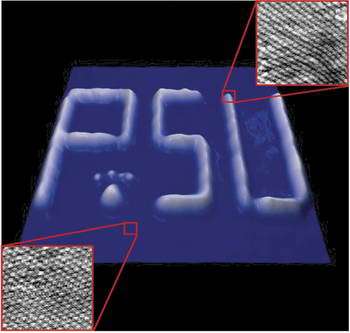
Image: The writing is less than half an Angstrom "tall" off the surface. The characters are less than 500 Angstrom high. They were created when Weiss and his team moved hydrogen atoms underneath a palladium surface using a custom-built, ultrastable, low-temperature, scanning tunneling microscope.
Observations of the effects of the resulting subsurface hydrides -- hydrogen atoms with a partial negative charge -- confirmed the existence of the stable sites, which had been predicted but previously had neither been deliberately assembled nor directly observed. The research was led by Paul S. Weiss, distinguished professor of chemistry and physics at Penn State.
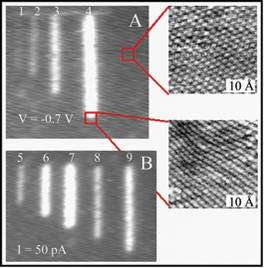
Right: Manipulation of subsurface hydorgen atoms in palladium by scanning tunneling microscopy to form the subsurface hydride.
After moving absorbed hydrogen atoms to just below the crystal surface, the researchers were able to observe how the presence of the hydride in specific sites within a metal crystal affects the chemical, physical and electronic properties of the metal. Understanding these effects could advance efforts to improve chemical reactions involving metal catalysts. In addition, the subsurface hydride may provide a model material for application in hydrogen storage and fuel cells. The ability to prepare the subsurface hydride provides an important research tool for these applications.
Weiss pointed out that hydrogen atoms just below the surface of the metal have been thought to be important in a number of chemical reactions. "Indirect experimental data have shown that chemically reactive hydrogen atoms were located at such sites, but there was no way to test them," said Weiss. "This material will allow us to test the predictions and to apply data from direct observation."
The researchers carried out the experiments in a low-temperature scanning tunneling microscope (STM) under ultrahigh vacuum by exposing the crystal to a hydrogen atmosphere. They removed excess hydrogen from the surface by cycles of exposure to heat and oxygen. After the surface had been cleaned, the researchers were able to use electrons from the STM tip to move hydrogen atoms that had been absorbed into the bulk metal up into the stable subsurface sites.
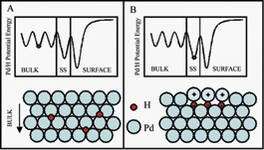
Right: Schematic of the effect of populating subsurface sites with hydrogen atoms from the bulk.
As the hydride formed underneath the surface of the material, Weiss and his team observed that the surface of the crystal distorted, the positive charge of palladium atoms above them increased, and interactions occurred with hydrogen atoms on the surface of the palladium crystal. "One of the most interesting aspects of the research was the ability to move atoms beneath the surface," Weiss said. "The observation of the effects of the populated sites, such as surface distortion, confirmed the existence of the stable sites and the theoretical predictions of the physical and electrical properties of the hydrides."
Years ago, Weiss was the first on an IBM team to manipulate xenon atoms on a metal surface. His coworkers later moved atoms to spell out their corporate logo. By extending the ability to manipulate atoms beyond the surface of a material, this research is expected to advance the understanding and control of important chemical reactions in a variety of commercial applications. In addition, this ability has potential as a model system of a technologically important material.
Source: Penn State