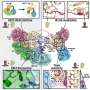
Do cells always have to be developed from organic carbon-containing compounds? When resourceful scientists stretch their imaginations, they quickly find an answer to this question. This is demonstrated by the work of Achim Müller of Bielefeld, Germany, and his co-workers, who have constructed an "artificial cell" from an inorganic macromolecule: a spherical polyoxymolybdate cluster.
Twenty round openings, each surrounded by an alternating series of nine molybdenum and nine oxygen atoms, form pores in the artificial cell membrane. Covalently bound in the interior are twofold negatively charged sulfate groups, which provide for a significant negative charge on the surface of the capsules. Water molecules are also found inside the sphere. Each pore is closed off by a “stopper” consisting of a urea molecule bound to the Mo9O9 ring by noncovalent interactions.
A typical example of biological signaling processes in living cells is a controlled ion flow through special channel proteins in the cell membrane. This can be controlled through the binding of a suitable ligand or by the electrochemical potential across the cell membrane, so ultimately by the difference in concentration of ions inside and outside the cell. Calcium ions (Ca2+) play an important role in many biological functions. For this reason, Müller et al. chose to use Ca2+ for their further experiments. They added Ca2+ ions to an aqueous solution of the molybdate capsules and examined the resulting crystals by X-ray crystal structure analysis, which revealed that not only did the calcium ions wander into capsules but that the urea stoppers were also back in place inside the Mo9O9 pores.
This behavior of the artificial cell mirrors events that unfold in a voltage-gated ion channel in a living cell. Initially, the pores are closed. When an excess of Ca2+ ions is added, their positive charges cancel out the negative charges on the surface of the sphere which changes the electrochemical gradient across the artificial cell membrane. The lids on the pores open, allowing Ca2+ ions to flow into the capsule. This possibly changes the charge distribution across the artificial cell membrane again such that the pores close up.
Source: Angewandte Chemie