Discovery of Ferroelectricity of Croconic Acid, a Low-molecular-weight Organic Compound, at Room Temperature

Sachio Horiuchi (Senior Research Scientist) of Correlated Materials Photoelectronics Group (Leader: Tatsuo Hasegawa), the Photonics Research Institute (Director: Masanobu Watanabe) of the National Institute of Advanced Industrial Science and Technology (AIST) (President: Tamotsu Nomakuchi); Yoshinori Tokura (Professor) of the Department of Applied Physics, Graduate School of Engineering and Ryo Shimano (Associate Professor) of the Department of Physics, Graduate School of Science, both of the University of Tokyo (President: Junichi Hamada); and Yusuke Tokunaga (Researcher) and Hiroki Itou (former Researcher, now Assistant Professor of Tohoku University) of the Japan Science and Technology Agency (JST) (President: Kouichi Kitazawa) have jointly discovered that croconic acid exhibits ferroelectricity and the largest polarization at room temperature among low-molecular-weight organic compounds. This study is a part of the "Tokura Multiferroics Project" of the Exploratory Research for Advanced Technology (ERATO) of JST (Research Director: Yoshinori Tokura).
Ferroelectrics are important materials and are used to perform various functions in the fields of both electronics and photonics; they are used in memory, capacitors, piezoelectric devices, and optical devices. The development of high-performance organic ferroelectrics is expected to encourage new applications utilizing the characteristics of organic compounds. However, the progress of organic ferroelectrics, particularly those made of small molecules, has been slow because very few organic ferroelectrics are known so far and they have low operating-temperatures and weak polarization properties.
Croconic acid has a simple molecular structure and contains carbon, hydrogen, and oxygen atoms (Fig. 1). We found that croconic acid is an excellent organic ferroelectric, and its spontaneous polarization is close to that of barium titanate, one of representative ferroelectric ceramics. In addition, the electric field required to reverse the polarity of croconic acid is significantly weaker compared to that required in the case of typical ferroelectrics made of organic polymers. The ferroelectric phase-transition temperature of croconic acid is over 150 °C, the highest value among the low-molecular-weight organic ferroelectrics, and croconic acid exhibits stable ferroelectricity at room temperature. The discovery that the organic compound known for over 180 years exhibits strong ferroelectricity suggests that other organic compound may have ferroelectricity as strong as those of inorganic ferroelectrics. Hence, this discovery is expected to encourage the development of ferroelectric organic materials.
The details of this research will be published in the February 11 issue of "Nature," an international scientific journal.
Ferroelectricity is the property of nonvolatile electric polarization that can be reversed by the application of an external electric field and the polarization is retained even after the field is removed. Ferroelectrics such as barium titanate, lead zirconate titanate, and polyvinylidene fluoride (PVDF) play important roles in electronics, are used to perform various functions, and have a wide range of applications such as in memory, capacitors, sensors, and piezoelectric and optical devices. Power-saving nonvolatile memory (FeRAM) is a typical example of those applications, and FeRAMs have become increasingly popular, e.g. they are used in IC cards. As new industrial applications are expected, there is a high demand for the improvement in performance of the devices taking advantage of organic materials that contain no rare metal or toxic lead and have properties including lightness, flexibility, and adaptability to large areas.
Polymers, such as PVDF, are well-known solid organic ferroelectrics. The polarity of the ferroelectric polymers is reversed when an electric field is applied because the field causes a rigid rotation of the substitution groups around the main chain; however, a very high driving voltage is necessary to induce the rotation. Therefore, in the case of organic ferroelectrics, reduction of the driving voltage, as well as the improved ability to store/accumulate large amounts of charge (spontaneous polarization,) is desired. The improvement in foundations for materials and molecular design of organic ferroelectrics including those made of low-molecular-weight compounds is then desired.
AIST proposed the original approach to ferroelectrics from acid-base supramolecules; using this procedure, organic ferroelectric materials with excellent polarization and dielectric constants (up to 2000 or more) at room temperature were developed. However, the problem with this approach is that an expensive deuteration process has to be carried out to achieve room-temperature operation. In addition to this problem, other difficulties characteristic of the multi-component systems may exist; for instance, the deterioration of their properties due to compositional changes and complicated manufacturing processes. Therefore, a new single-component material has been explored in this study, and an organic ferroelectric compound with the best spontaneous polarization at room temperature has been discovered. Theoretical calculations were performed in collaboration with the Institute of Condensed Matter Physics of the Italian National Research Council (CNR/INFM).
This study is supported by a Grants-in-Aid for Scientific Research (new frontier of materials science opened by molecular degrees of freedom, 2008-2012) of the Ministry of Education, Culture, Sports, Science and Technology.
Croconic acid, whose name is derived from its yellow color, is an organic compound that was discovered approximately 180 years ago. In recent times, it is used to synthesize croconium dye, which is a near infrared dye, and is available commercially. Croconic acid is composed of carbon, hydrogen, and oxygen atoms and its molecular structure has a simple pentagon of five carbon atoms, two proton-donating hydroxyl groups (-OH) and three proton-accepting carbonyl groups (>C=O) (Fig. 2c).
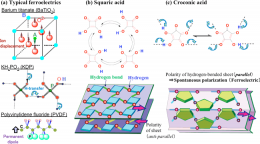
Figure 2 shows the chemical and crystal structures of croconic acid in comparison with those of squaric acid (not ferroelectric) and conventional ferroelectrics. Both squaric acid and croconic acid have a sheet structure with strong intermolecular hydrogen bonds. They show a characteristic reversal of the polarity of each sheet due to the shift in the position of double bonds; this shift accompanies the proton transfer from the hydroxyl groups of one molecule to the carbonyl groups of an adjacent molecule. This mechanism differs from the mechanism of ion displacement in barium titanate (BaTiO3) and that of rigid rotation in PVDF; rather, the mechanism of the reversal of polarity in croconic acid is similar to that of potassium dihydrogen phosphate (KH2PO4); ferroelectricity of KH2PO4 is a result of the movement of protons in the hydrogen bonds between PO43- ions (Fig. 2a). In the case of squaric acid, spontaneous polarization (ferroelectricity) is lost because of the cancellation of the polarities of adjacent sheets. In contrast, in the case of croconic acid, we presumed that the ferroelectricity is retained because the polarities of the sheets do not get cancelled.
To measure the properties of croconic acid including ferroelectricity, large high-quality single crystals of croconic acid were grown (Fig. 1). A large spontaneous polarization of 21-22 μCcm-2 was observed on the application of an electric field parallel to the hydrogen bond chains (i.e., perpendicular to the most developed surface of the crystal). This is close to the theoretical value (26 μCcm-2) calculated using the first-principles electronic-structure calculations based on the crystal structure model by CNR/INFM. The measured value is significantly higher than the current highest value of polarization observed in organic ferroelectric materials (12-13 μCcm-2 of PVDF or its oligomers) and is close to that of barium titanate (26 μCcm-2), which is a typical ferroelectric material used in ceramic capacitors. In the polarization-electric field hysteresis curve, the electric field required for reversal of polarity (Ec in Fig. 3a) is approximately 15 kVcm; in other words, the electric field required for croconic acid is smaller than that for the ferroelectric polymers by 1-2 orders of magnitude. The large spontaneous polarization was not decayed at all until approximately 130 °C (400 K) (Fig. 3b), and the phase-transition temperature (Curie point), at which ferroelectricity is lost, was not observed below 150 °C. Both the polarization value and the Curie point are extremely higher than those of the conventional ferroelectric materials made of low-molecular-weight organic compounds, as shown in Fig. 3c.

Owing to the high spontaneous polarization, i.e. the density of charges that can be stored on the material surface is high, the cell size of nonvolatile memory can be minimized. At the same time, the high spontaneous polarization is a starting point to enhance the properties of ferroelectric materials, for example, permittivity for capacitor function, pyroelectricity for the detection of heat and temperature changes, and piezoelectricity for interconversion between electric energy and mechanical energy.
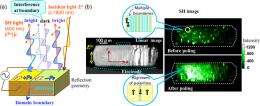
In addition, the large polarization resulting from the alignment of molecular polarities in the crystal by an external electric field (poling), has been proved by observing the second-order nonlinear optical effects; the incident laser light (800 nm wavelength) generates light (second harmonic light: SH light) of frequency twice (400 nm) that of the incident light, as shown in Fig. 4. Figure 4b shows the two-dimensional images of SH light intensity of a sample observed by a nonlinear optical microscope. Before poling, the random distribution of both polarized areas (domains) of upward and downward polarization creates dense domain boundaries, and interference at the domain boundaries weakens the SH light intensities. By poling with the electric field, the area of domains polarized along the direction of the electric field is increased. With reduced density of the domain boundaries, the intensity of the SH light increases. The SH light maintains the intensity even after the field is removed, implying the remanence of aligned polarization.
In this study, a well-known low-molecular-weight organic compound with a simple chemical structure is found to exhibit remarkable ferroelectricity, indicating the possibility that other low-molecular-weight organic compounds may have the excellent ferroelectricity comparable to those of inorganic ferroelectric materials. Among the large number of organic compounds, many are expected to exhibit ferroelectricity, and hence, this discovery will greatly encourage the development of ferroelectric organic materials.
Provided by AIST