Application approval of new anti-influenza virus drug
Toyama Chemical Co., Ltd., a subsidiary of FUJIFILM Holdings Corporation, announced today that the Japanese Ministry of Health, Labour and Welfare has approved the New Drug Application of "Avigan Tablet 200mg" (Generic name: favipiravir; Development code: T-705; " Avigan") for a new tablet-format anti-influenza drug.
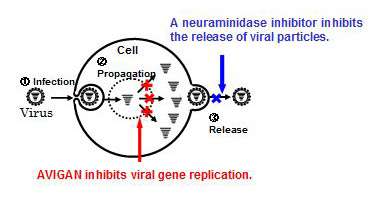
Influenza viruses replicate their genes within infected cells to propagate and release new viral particles and to spread the infection to other cells. Neuraminidase inhibitors, typically used in influenza treatment today, inhibit the release of new viral particles to prevent the spread of infection.
In contrast, Avigan is a viral RNA polymerase inhibitor with a new mechanism of action, inhibiting viral gene replication within infected cells to prevent the propagation.
Due to this characteristic, the drug is expected to have an antiviral effect on Avian Influenza A (H5N1 and H7N9) and other viruses, with efficacy already confirmed in animal studies.
Avigan has obtained the approval in Japan ahead of other countries after the authorities considered current situations of influenza and recognized significance in making Avigan, which has a new mechanism of action, available to establish preparedness against the possible outbreak of novel or re-emerging influenza virus infections, to which neuraminidase inhibitors or other anti-influenza drugs could be ineffective or not sufficiently effective.
Avigan is a pharmaceutical product that is to be administered to those infected with novel or re-emerging influenza viruses when the government makes a decision to use it to control such viruses. It is therefore manufactured and distributed upon request by the Minister of Health, Labor and Welfare, rather than immediately being marketed to healthcare providers.
Toyama Chemical will establish a stable supply structure to be activated upon request from the Minister of Health, Labor and Welfare, by paying full considerations for distribution management and safety measures.
Provided by Fujifilm