Inhibitory synaptic plasticity in the cerebellum contributes to motor learning
The research group lead by Tomoo Hirano (Professor, Graduate School of Science) and its member, Shinsuke Tanaka (Graduate Student) et al. found that synaptic plasticity at cerebellar inhibitory synapses is involved in motor learning.
This report was published in the "Journal of Neuroscience" on October 23, 2013.
Background
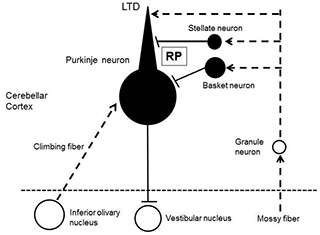
Neurons in the central nervous system transmit information through synapses, and the transmission efficacy changes depending on the activities of neurons. This phenomenon is called synaptic plasticity, and has been regarded as a critical mechanism of learning and memory. The cerebellum is essential for fine motor control and is involved in motor learning. In particular, a type of synaptic plasticity, long-term depression (LTD) at granule neuron to Purkinje neuron excitatory synapses (Fig.1) has attracted much attention as a primary cellular mechanism for motor learning. LTD is the depression of synaptic transmission efficacy induced by co-activation of a granule neuron and an inferior olivary neuron, both forming excitatory glutamatergic synapses on a Purkinje neuron. However, recent scientific papers reported normal motor learning with suppressed LTD, which suggested other cellular mechanisms may be also important for motor learning.
Previous studies showed that synaptic plasticity also occur at inhibitory synapses between a stellate neuron and a Purkinje neuron. Here, activation of an inferior olivary neuron enhances this GABAergic inhibitory synaptic transmission for long-term. This synaptic plasticity is called rebound potentiation (RP) (Fig.1). Physiological roles of RP had been unknown. We thought RP might work together with LTD for establishment of motor learning, because activation of an inferior olivary neuron is required for both LTD and RP, and also because both down-regulation of excitatory synaptic inputs by LTD and up-regulation of inhibitory synaptic inputs by RP work to suppress the Purkinje neuron activity. To test this idea, we generated transgenic mice defective in RP.
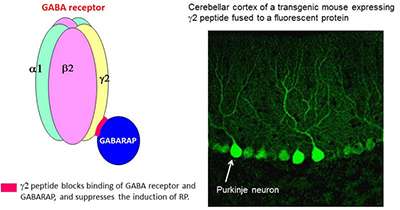
The group's previous study revealed that binding of GABA receptor and an intracellular protein GABARAP is necessary for RP, and that a peptide (γ2 peptide) which blocks this binding suppresses the RP induction (Fig.2). To address functional roles of RP, we generated transgenic mice that express γ2 peptide fused to a fluorescent protein only in Purkinje neurons (Fig.2). The transgenic mice did not show RP as we expected, and other physiological and morphological properties of the cerebellum appeared normal.
Results
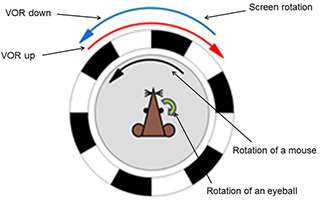
Then the group evaluated the motor control and learning ability of the transgenic mice by examining vestibulo-ocular reflex, which works to stabilize the visual image during head motion. Vestibulo-ocular reflex undergoes adaptive modifications in direction to reduce the image slip on a retina, regarded as a model paradigm of cerebellum-dependent motor learning. In experiments, a mouse was rotated sinusoidally, and a surrounding external screen with vertical black and white stripes was also rotated simultaneously. When the screen rotation was in the opposite direction to the mouse rotation, the gain of VOR increased gradually in a normal wild-type mouse, and when the rotations were in the same direction, the gain decreased. These changes of VOR in a wild-type mouse was in the direction to reduce the image motion on a retina and adaptive (Fig.3). These adaptive modifications were clearly suppressed in the transgenic mice defective in RP.
In conclusion, transgenic mice defective in RP were generated and showed defects in a type of motor learning. These results indicate that RP, a type of synaptic plasticity at inhibitory synapses in the cerebellum, contributes to motor learning.
More information:
dx.doi.org/10.1523/JNEUROSCI.0793-13.2013
Provided by Kyoto University