Decoding protein structures helps illuminate cause of diabetes
Any photographer can vouch for the difficulty of capturing a clear picture of a moving target. When it comes to molecules, however, sometimes the motion is exactly what scientists want to see - for example, to understand the pathological protein mis-folding and assembly that seem to underlie a host of human disorders, including diabetes and Alzheimer's disease.
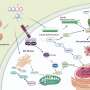
Now, chemists at the University of Wisconsin-Madison have designed a powerful analytical tool capable of measuring molecular structures quickly and accurately enough to catch moving proteins in mid-fold and see the shapes of intermediate steps. As described in this week's online issue of the Proceedings of the National Academy of Sciences, the first applications of the technique offer a glimpse into the contorted form of a human protein that is implicated in type II diabetes.
Pancreatic damage in type II diabetes has been linked to toxic clumps of the protein hIAPP (human islet amyloid polypeptide), which is normally produced by the same cells that make insulin. An unknown trigger prompts the protein to fold into sharp fibers that poke holes in pancreatic cells, killing them.
Though scientists already have a good idea of the healthy "before" and dangerous "after" hIAPP structures, the steps in between remain somewhat of a mystery and may hold clues to what drives the transition, says UW-Madison chemistry professor Martin Zanni, who led the new study.
In trying to understand diabetes, "people have been looking at the fibers, but they should be looking at their formation," he says. "Somehow that mechanism is causing holes [in pancreas cells], which cause disease."
To break down this dynamic process, he adds, "We need tools that not only allow us to probe the molecular structures, but also look at how the structures change in time."
Zanni's research group uses a method, known as two-dimensional infrared spectroscopy (2-D IR for short), that takes advantage of the restless nature of molecules and atoms.
Though often depicted as static blobs, proteins are more like collections of balls and springs, constantly in motion, and their endless atomic twitching conveys information about their organization, Zanni says. Infrared laser beams can detect the minute vibrations and identify characteristic patterns to deduce protein structures.
A few years ago, Zanni's team built the first device capable of designing infrared laser beams with a computer. The team has now simplified and speeded up the process with an automated version of the 2-D IR technique. As described in the current study, they obtained a single structural scan of hIAPP in less than a second - more than 500 times faster than previously possible.
The speed is crucial for trying to understand a dynamic process like hIAPP mis-folding, Zanni says.
The group now plans to capture series of snapshots during individual folding reactions to identify multiple phases as the proteins convert from an unordered mishmash into flat sheets, then coil into fibers.
"No matter how fast they're moving, we can take pictures of them," says Zanni. Without their automated method, he says, such experiments would be nearly impossible.
The technique also has potential application in other human diseases that involve protein mis-folding, such as Alzheimer's and Huntington's diseases.
At this time, however, he says the automation of the device itself is a tremendous achievement. "In time, automated 2-D IR spectroscopy will become a common analytical technique, widely available in university and industrial research laboratories around the world."
Source: University of Wisconsin-Madison