Diffraction and scattering -- the solution to what's in solution
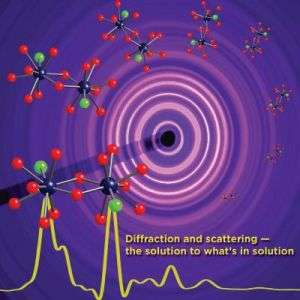
Researchers at the Department of Energy’s Argonne National Laboratory and the University of Notre Dame have successfully applied X-ray scattering techniques to determine how dissolved metal ions interact in solution.
These findings will help researchers better understand how metal ions, such as those found in nuclear waste and other industrial processes, behave in the environment.
The results show that the ion structures are visible in solution and reveals their interactions with other ions.
"The scientific community has long asked the question, 'What happens to a metal ion in solution?'” said Suntharalingam “Skantha” Skanthakumar, Argonne senior scientific associate. "Direct measurement of metal correlations in solutions show long-range interactions and a strong correspondence to the structures in solution and solid state environment."
"We have been provided with additional structural and chemical insight into tetravalent actinide hydrolysis," said Lynda Soderholm, senior scientist at Argonne. "We discovered that the way atoms interact is transferable with a lot more detail than what was previously thought. Hydrolysis of dissolved metal ions is one of the most fundamental and important reactions in aqueous chemistry.”
Experiments for this work were conducted at Argonne’s Advanced Photon Source (APS). The 1,104-meter circumference APS accelerator complex, large enough to encircle a baseball stadium, houses a complex of machines and devices that produce, accelerate and store a beam of electrons that is the source of the APS X-rays. For this research, thin beams of high-energy X-rays were used to bombard the dissolved ions. When the X-rays scattered off the solutions, special CCD cameras equipped to detect them mapped out their two-dimensional pattern.
The detailed results of these findings were published in the paper, "Structures of Dimeric Hydrolysis Products of Thorium" and in the journal Inorganic Chemistry.
"Going forward, additional research is planned with thorium and other dissolvable materials across the periodic table," said Argonne postdoctoral researcher Richard E. Wilson. "The goal is to be able to predict reactions to metal contaminants and determine the chemistry that influences their transport in the environment"
This research involved collaborations from various scientific disciplines including input from physicists, chemists and geologists.
Source: Argonne National Laboratory