Researchers discover variants of natural tumor suppressor
Building on their 2005 discovery of an enzyme that is a natural tumor suppressor, researchers at the University of California, San Diego School of Medicine have now identified two variants of that enzyme which could provide new targets for therapies to treat diabetes, heart and neurological disease. The findings, by Alexandra C. Newton, Ph.D., UCSD professor of pharmacology, and colleagues are published in the current edition of the journal Molecular Cell.
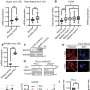
Previous research by Newton's lab, also published in Molecular Cell, described the discovery of an enzyme they named PH domain Leucine-rich repeat Protein Phosphatase (PHLPP, pronounced "flip") that turns off signaling of the Akt/protein kinase B, a protein which controls cell growth, proliferation and survival.
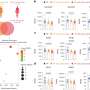
The new work describes a second family member, PHLPP2, which also inactivates Akt, inhibiting the cell cycle progression and promoting cell death. However, PHLPP1 and PHLPP2 control three different disease pathways. While both are important in cancer, PHLPP 1 impacts an important pathway in diabetes and PHLPP2 could be useful in fighting heart and neurological disease.
"We first discovered that PHLPP controls Akt, which is the driver on the pathway to tumor growth," said Newton. "PHLPP is like a brake that, when on, slows the driver but when 'off' allows the driver to move. In cancer, we want the driver to brake, to prevent cell proliferation leading to tumor growth. But in diabetes, heart or neurological disease, where we want to promote cell growth and survival, we don't want to slow the driver down."
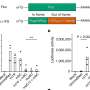
The researchers have now found that PHLPP1 controls the driver along one pathway – Akt2, which is more closely involved in maintaining a constant level of glucose in the bloodstream. Therapies directed at inhibiting PHLPP1 could be used to treat diabetes; in essence, removing the 'brake' and allowing Akt2 to be more functional and allow better insulin regulation. PHLPP2, on the other hand, controls the driver on Akt1, the path involved with cell survival. Therapies directed at releasing the brake on this driver would allow cells involved in heart or neurological diseases to better survive.
"Both PHLPP variants are important in cancer; the loss of a brake to any of the three Akt pathways sends 'go, go, go' signals that promote the survival of tumor cells," said first author John Brognard. UCSD researchers had previously discovered that Akt is hyperactivated, or elevated, in most cancers and PHLPP provides a mechanism to reverse this activation.
Source: University of California - San Diego