Mixed metals not so mixed up at the nano-level
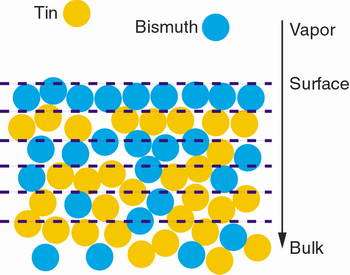
With the help of the world's most brilliant hard X-ray beams at the Advanced Photon Source, scientists have seen for the first time metal atoms near the surface of a liquid alloy arrange themselves in alternating layers one atom thick.
Image: Scientists have seen for the first time metal atoms near the surface of a liquid alloy arrange themselves in alternating layers one atom thick.
The research has implications for nanomaterials — materials made of ultrafine particles in which most atoms lie at or near the surface — and could lead to such applications as improved lead-free solder for electronics.
The collaboration, led by physicists from Harvard University, used X-rays to look at how atoms of bismuth and tin behave when they form a liquid alloy. In the bulk material, the two elemental liquids form a perfectly miscible solution — like cream mixed with coffee — but near the surface they separate into atomic layers with alternating compositions.
"The top atomic layer is mostly bismuth," said Oleg Shpyrko (currently at Argonne's Center for Nanoscale Materials, or CNM), the lead author of the study. "Below that layer, it's mostly tin. The layers alternate as you get deeper into the material, but after a few layers they start to mix until it becomes a pure alloy."
Previous studies have observed the low-surface tension component in such a mixture form a surface monolayer, a phenomena known as Gibbs adsorption. However, the extension of surface effects to sub-surface layers has never before been observed.
"The demixing we observe is somewhat of a paradox, since the interactions between the two components are strongly attractive, not repulsive, as in the case of immiscible mixtures like oil and water," Shpyrko said. "Surface demixing was predicted in 1950, but it eluded experimentalists for more than 50 years."
Shpyrko and collaborators developed an X-ray technique that allows independent measurements of atomic structure in the near-surface region of the liquid. The technique can also determine, and allow the researchers to compensate for, the effects of temperature-induced surface waves. These fluctuations can obscure direct observation of surface structures on minute scales.
The researchers have learned that surface-induced layering is a quasi-crystalline structure that appears at liquid-vapor interface of even simplest metallic fluids, but is apparently absent in dielectric liquids such as water.
In the most recent study, Shpyrko and co-workers added resonant X-ray scattering to obtain element-specific density profiles, while retaining sub-nanometer spatial resolution — a method that can be applied to a wide range of multi-component liquids in the future studies.
"As we learn about a variety of novel nanoscale materials where most atoms are near the surface, these and other interfacial effects are expected to play a dominant role," Shpyrko said.
The study was primarily led by Harvard's Peter Pershan and his group members Alexei Grigoriev, Reinhard Streitel and Diego Pontoni. Contributing were Ben Ocko from Brookhaven National Lab, Moshe Deutsch from Bar-Ilan University in Israel, and Binhua Lin and Mati Meron, who provided beamline support at The University of Chicago's ChemMat-CARS facility at the Advanced Photon Source.
The results were reported in Physical Review Letters [Phys. Rev. Lett. 95, 106103 (2005)]
Source: ANL (Dave Jacque)