Nano design adjustment may help find, clear some water contaminants
Experiments designed to test discrepancies in theoretical computational chemistry have turned up a barely two-angstrom difference that may lead to a new approach to locate and remove dangerous toxins such as perchlorate and nitrates from the environment.
The research targets toxic groundwater contaminants that contain negatively charged ions known as anions (a-NI-ens), which are historically difficult to remove. Perchlorate, a rocket fuel additive recently linked to thyroid deficiency in women, has contaminated more than 450 wells in California alone. Nitrate contamination, which results mainly from the use of nitrogen fertilizer, is a leading cause of shutdowns of wells and public water supplies in the United States.
"There is a need for improved materials that are effective at removing anions from the environment," said Darren W. Johnson, a University of Oregon chemist and co-principal investigator of a study appearing online Dec. 13 ahead of regular publication in the Journal of the American Chemical Society. "A current leading strategy is anion exchange, which uses a polymeric resin to exchange an anion for one that’s not a problem." (Two other currently used methods aimed at anions are biochemical denitrification and reverse osmosis.)
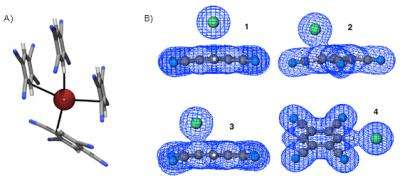
In the new study, led by UO doctoral student Orion B. Berryman, researchers focused on anion-pi interaction, in which a negatively charged species is attracted to a neutral electron-deficient aromatic ring, which could be incorporated into a specifically designed receptor.
Anion-pi interactions have been the focus of recent theoretical work, in which electronic structure calculations predicted that anion binding between halides and electron-deficient aromatic rings will occur over the center of a ring. However, the lab experiments on crystalline material found that the binding occurs as much as 2 angstroms, or 0.2 nanometers from the center.
"It's very important to consider these off-centered anion-interactions occurring through a charge-transfer interaction," Berryman said. "We looked at solid-state structures and the geometry of the interaction involved in a simple system. In these initial studies we noted significant color changes due to this off-center binding geometry found in the crystal structures."
Co-principal investigator Benjamin P. Hay, a chemist at the Pacific Northwest National Laboratory in Richland, Wash., where Berryman studied last fall as part of UO's National Science Foundation-funded internship program, said the study has important ramifications in anionophore design, crystal engineering and other aspects of supramolecular chemistry. In fact, he said, the findings indicate that prior designs may be flawed, incomplete or even misleading. "We discovered an unexpected bonding motif that involves the transfer of charge from the anion to the arene -- in other words, a covalent bonding motif," Hay said. "This is the first theoretical characterization of what we have termed an off-center, weak charge-transfer interaction."
Anions, of which notable examples include DNA, nitrate, pertechnetate, cyanide and chromate, play indispensable roles in biological and chemical processes, but they also can contribute significantly to environmental pollution that threatens aquatic life cycles and human health.
Johnson, in collaboration with UO chemist Michael M. Haley, now is seeking to design receptors that aim to the off-center location, with a goal of developing sensors for anion detection. Because Berryman's research produced sometimes intense color changes at binding sites, such an approach could lead to developing materials that sense the presence of these toxins and remediate them.
While 0.2 nanometers seems an insignificant distance, it could mean there's a 100 percent chance that binding cannot occur, Johnson said. "We're finding that from a design standpoint, that 0.2 nanometers is a big difference."
He noted that estimating or calculating the binding distances when optimizing a receptor for positively charged binding, or cation, such as the chelation of metals by EDTA (ethylenedinitrilotetraacetic acid), is done almost exactly --s (0.01 nanometers). EDTA is widely used in industrial cleaners, detergents and textile production.
Source: University of Oregon