Measuring Synthesis Intermediates for Better Materials
Involved in about 90 percent of all chemical processes and the creation of about 60 percent of the chemical products available on the market, catalysis is vital to American industries. Catalysis, the acceleration of a chemical reaction by means of a separate substance (the catalyst), benefits fields such as chemistry, petroleum production, environmental protection, pharmaceuticals and bioengineering and the development of fuel cells.
New information about catalysis can lead to better and more efficient materials and processes such as oil refining and reducing harmful emissions in motor vehicles. But while catalysis might sound like it’s all about the end product, Brookhaven National Laboratory and University of Connecticut researchers are focused on what happens in between.
The properties and activity of materials can vary significantly with different synthesis methods or at different conditions, such as temperature and time. Using x-rays at beamline X7B of the National Synchrotron Light Source, BNL and UConn researchers measured the changes catalyst materials go through during synthesis, showing that kinetic and mechanistic information about certain materials could allow for better synthesis control. “By understanding the intermediate, we can define the conditions whereby you get the product you want,” said BNL chemist Jon Hanson, part of the group that performed the research.
The traditional way of making these observations is done outside of the reaction environment, termed ex-situ. During this method, target materials are separated from the reaction systems after different reaction times and then washed and dried before structural analysis. However, this process can alter materials from their original state in the reaction systems.
“You’re probably going to change the material when you measure it,” Hanson said. “You can’t observe the characteristics under processed conditions.”
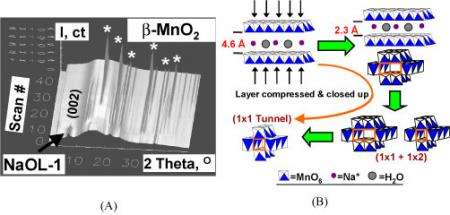
Thanks to high-flux x-rays, scientists can avoid that alteration with a method called in-situ synchrotron x-ray diffraction. This process provides a real-time look at the phase transformations involved in the synthesis or catalysis without removing them from the reaction environment. At the NSLS, Hanson together with Xiong-Fei Shen from Steven Suib’s research group at the University of Connecticut used this mode to study structural changes during the synthesis of manganese oxide octahedral molecular sieves, a group of materials that have uses ranging from catalysts to gas sensors.
To find out details about manganese oxide synthesis, a different type of in-situ procedure was used. The majority of in-situ studies use the targeted material in a solid or gel form. BNL and UConn researchers, however, used hydrothermal synthesis, in which the material is in a heterogeneous liquid-solid form. Researchers heated a slurry of dried birnessite, a layered-structure manganese oxide, with HNO3 solution. They then “watched” the effects of different temperatures on the mixture through x-ray diffraction and determined the conditions needed to obtain different structures of the compound. For example, after heating the mixture for 15 minutes at 180 degrees Celsius, a 1 x 2 tunnel structure of manganese oxide starts to form. Similar observations were performed with KOMS2, for which scientists determined the conditions needed to produce desired surface areas. Knowing basic characteristics, such as structure and surface area, as materials are synthesized can provide better control or “tuning” to get the catalytic properties scientists and industries want, Hanson said.
The results of their work are published in the April 12, 2006 edition of the Journal of the American Chemical Society. Other scientists involved in this research include Yun-Shuang Ding, and Mark Aindow (University of Connecticut).
Source: Brookhaven National Laboratory , by Kendra Snyder