Uniform tungsten trimers stand and deliver
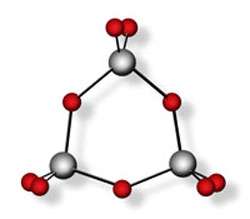
Like tiny nano-soldiers on parade, the cyclic tungsten trioxide clusters line up molecule-by-molecule on the titanium dioxide platform. One tungsten atom from each cluster is raised slightly, holding forth the potential to execute catalytic reactions.
The nanostructures constitute a new model system, a simplified version of a catalyst that would be used in an application. Such models reveal to chemists the exact structure and reaction mechanisms of metal oxides.
Developed by researchers from the Department of Energy's Pacific Northwest National Laboratory, the University of Texas-Austin and Washington State University, the discovery may offer a platform for fundamental reactivity studies of metal oxides used as catalysts in converting hydrocarbons into fuels and value-added chemicals.
"There is a striking difference between commercial catalysts and the new model system," said Mike White, the UT professor leading the PNNL Institute for Interfacial Catalysis. Variability in commercial catalyst size and chemical composition makes it difficult to accurately understand or describe the reactions taking place at a molecular level.
"Commercial catalysts are like a gravel pile with many sizes of rocks. Some rocks are purple; some are blue. Some do one thing; some do another. But, our system has all the same size rocks," White said.
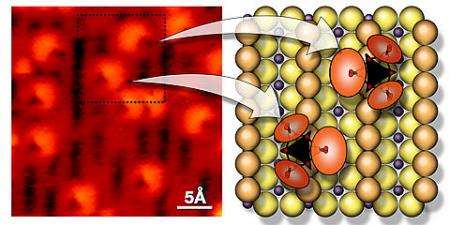
The model system – in which all the molecular clusters are the same size, are evenly dispersed and are oriented in one of two directions on a single layer of titanium oxide crystals – holds promise as a platform for studying the behavior of early transition metal oxides. White noted, "While we have created the smallest nano-cluster of a uniform size you can imagine, it is a real oxide. The tungsten is in its normal oxide state. In principle, you have all the things needed to make bonds and break bonds. That's the scientific breakthrough here."
Though it appears simple, the model system was challenging to develop, White said. The collaborators employed specialized equipment available from the William R. Wiley Environmental Molecular Sciences Laboratory, a DOE user facility located at PNNL, to prepare and characterize the platform as well as the clusters. Using a unique approach that changed the tungsten oxide directly from a solid to a gas, the collaborators stabilized the molecular rings – or "trimers" – of tungsten on the titanium platform.
A scanning tunneling microscope imaged not only the trimers but also their consistent alignment with the single crystal structure of the platform. "A scanning tunneling microscope must be so stable that we have to vibrationally isolate the instrument. It cannot move even a small amount because we are using a stream of electrons to measure the distance from the microscope's tip to a small space between atoms," White explained. The collaborators also characterized the cluster mass, determined the ratio of tungsten to oxygen atoms in the cluster, and used X-ray photoelectron spectroscopy to identify the tungsten oxidation state.
"This is a small piece of the basic science that could lead to control of chemical transformations for our energy future," White said, noting that this is the first time researchers have created and imaged monodisperse oxide clusters on another oxide.
Source: Pacific Northwest National Laboratory




















