Nanotubes in a New Light
Using x-rays to investigate order and function in nanotube systems
Nanotubes are tiny cylindrical molecules just a few nanometers in diameter, but their potential for new technologies is vast. They are extraordinarily strong, conduct electricity well, and can even emit light, properties suitable for many applications, from flat-panel television displays to fuel cells to building materials. But nanotubes must be extensively studied before they can be used in industrial applications.
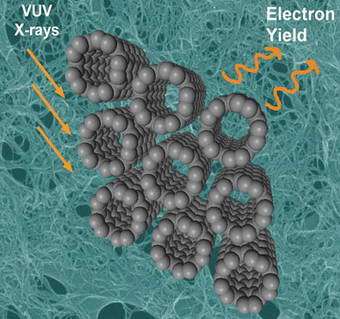
Image: A rendering of carbon nanotubes being studied using NEXAFS. Light comes in (left) and electrons are emitted (right).
In collaborative research, a team of scientists has pioneered a novel way of using x-rays at the NSLS to study arrays of nanotubes. In an ongoing series of research projects, they have determined the degree of order contained in certain nanotube systems - that is, to what extent they form organized patterns - and have investigated the structural and chemical properties of others.
The collaboration team includes Mahalingam Balasubramanian, Brookhaven National Laboratory (BNL); Sarbajit Banerjee, Stony Brook University (SBU); Tirandai Hemraj-Benny, SBU; Daniel Fischer, National Institute of Standards and Technology (NIST); Weiqiang Han, BNL; James Misewich, BNL; Sharadha Sambasivan, NIST; and Stanislaus Wong, BNL and SBU.
The group is investigating assemblies containing two types of carbon nanotubes: single-walled nanotubes (SWNTs), consisting of a single-shelled cylinder, and multi-walled nanotubes (MWNTs), which resemble cylinders concentrically nested together like Russian dolls. They have also completed a study of nanotubes composed of a different, yet equally intriguing material, boron nitride, which is composed of the elements boron and nitrogen.
A New Approach
Nanotubes are being studied by many scientists across the globe, and the technique used in this case has been used for years to study various materials. Now, several aspects of this technique have been applied in novel ways to nanotubes, with excellent results.
The synchrotron-based technique is known as "near-edge x-ray absorption fine structure," or NEXAFS. In NEXAFS analysis, each nanotube sample is placed in the path of a beam of low-energy x-ray "photons," or particles of light. The x-ray photons are absorbed by each carbon atom's "core" electrons - those closest to the nucleus - giving them an energy boost. As a result, they jump to an orbit further away from the nucleus. When this occurs across many, many atoms, scientists can record the sample's absorption "spectrum," which measures the absorption behavior of the sample based on specific energies of the x-ray photons.
There are two ways to measure the absorption spectrum: first, by measuring the photons emitted when the energized electrons de-energize and fall back to a lower orbit; or, in certain cases, by measuring electrons that are emitted.
At beamline U7A (owned by NIST and The Dow Chemical Company), the researchers aimed the x-rays at each sample from several angles, producing several spectra for each array. By analyzing the spectra, they uncovered information about the nanotubes' electronic and physical structures.
"The beauty of using NEXAFS to study nanotubes is that it is able to provide us with information that is truly complementary to what can be obtained using other techniques," said Fischer. "Moreover, we are able to gather more detailed information than these other methods are capable of."
Your Order, Please
The group's most recent results on carbon nanotubes appear in the May 5, 2005, issue of the Journal of Physical Chemistry. The paper describes how the researchers used NEXAFS to determine the degree of order in very thin films of single-walled nanotubes, known as "buckypaper," and a MWNT film grown on a surface of platinum metal. They compared their results to two "control" groups: graphite, a highly ordered form of carbon with well-understood electrical and structural properties, as well as SWNT and MWNT powder samples, which have essentially no order.
The group found that the buckypaper spectra are similar to graphite's in that, for both, the spectra change when the x-rays graze the sample rather than strike head-on. This property - the tendency of a material to react differently to outside fields depending on the direction the field is applied - is called "anisotropy." It is a very useful behavior to study, since high anisotropy often implies a high degree of order, and is often a desirable property for nanotube systems to have. Conversely, a material shows "isotropy" when its reaction to an outside field is the always the same, regardless of direction. Nanotube powders, containing nanotubes in all possible positions and orientations, display isotropy. Scientists often speak of anisotropy and isotropy when they are discussing order in a system.
In buckypaper, the group found, the anisotropy is not as pronounced as in graphite. They explored this result with further analysis, and calculated that approximately 87 percent of the nanotubes in the paper lie on their sides. Thus, the paper isn't as ordered on the nanoscale level as it could be.
The analysis of the MWNT film is more preliminary, but shows that the film behaves as if most the nanotubes stand upright. Thus, the film also displays anisotropy.
"These results are encouraging because they indicate that two common nanotube systems already contain a fair degree of order," said Wong. "Moreover, for both the arrays and the paper, the results were stunningly close to what our preliminary theoretical calculations predicted."
Wong continued, "We plan to continue studying MWNT films grown on surfaces other than platinum, and will also look at single-walled nanotube films grown on various surfaces. Our hope is to find a way to produce a nanotube array with order comparable to graphite."
A Matter of Function
"Functionalizing" a set of nanotubes is an important process that allows scientists to manipulate the tubes into ordered arrays that have specific properties. One way to do this is "ozonolysis" - causing the nanotubes to react with ozone, a highly reactive form of oxygen gas. The reaction "drills" tiny holes into the nanotubes' sidewalls and creates various oxygenated compounds along the nanotubes' surface, which, in turn, serve as tethering points for a variety of other chemical compounds.
Ozonolysis can also cleanse the nanotubes, remove impurities, or produce reactions that "unhinge" the nanotubes' half-sphere end caps, leaving the hollow inside of the tubes accessible to a variety of interesting "stuffings." Filling the tubes with metal atoms, for example, could boost their ability to conduct electricity.
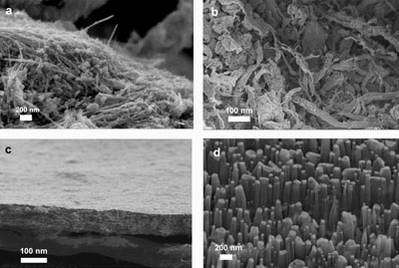
Image: Scanning electron microscope images of (a) MWNT powder, (b) SWNT powder, (c) SWNT buckypaper, and (d) aligned MWNTs.
But studying the specific electronic structure of the nanotubes, as well as the chemical make-up and ordering of the oxygen-carbon compounds, which are called functional groups, has proven to be difficult. Most techniques, including using infrared light and other conventional methods, are unable to simultaneously yield both types of information. The team showed that NEXAFS is an important complement to these other methods in characterizing nanoscale samples.
They have published two studies so far on their analysis of functionalized carbon nanotubes. The most recent, in the September 17, 2004, issue of ChemPhysChem, focuses on MWNTs.
When MWNTs were exposed to ozone and then analyzed with NEXAFS, the researchers confirmed many expected results. The ozone treatment cleared the nanotubes of "amorphous" carbon - that is, carbon atoms not incorporated into the ordered structure of the nanotubes themselves - opened the end caps, and produced functional groups along the nanotubes.
In the first publication, found in the April 7, 2004, edition of Chemical Communications,the group reported similar results for a NEXAFS analysis of the ozonolysis of SWNTs. They were able to track how exposure to ozone disrupted the distribution of electric charge on the nanotubes, as well as to determine which types and quantities of functional groups were formed.
These results show that NEXAFS is a very effective tool for gathering information on both types of carbon nanotubes. It allowed them to study the nanotubes' structure and, at the same time, gather data on the chemical composition and distribution of the oxygen-carbon groups.
A Nanotube by Another Name
The word "nanotube" is very often preceded by the word "carbon," but nanotubes made of other materials can be equally - or even more - interesting. One such material is boron nitride.
Boron nitride nanotubes are tremendously strong, just like their carbon counterparts. Moreover, they possess superior properties, such as flexibility without compromising strength and the ability to withstand very high temperatures. They are being investigated for a wide array of potential applications, such as high-temperature transistors, high-temperature lubricants, photoluminescent devices, reinforcements for weaker materials, and flat-panel displays.
In the February 17, 2005, online issue of the journal Physical Chemistry Chemical Physics, the team describes how they used NEXAFS to gain valuable structural information about a sample of boron nitride nanotubes made at Brookhaven Lab.
They demonstrated that the NEXAFS technique is very effective at yielding structural information on boron nitride nanotubes. Their results show that the boron nitride nanotubes in their sample have atomic structures that mimic a particular type of carbon nanotube, in which the carbon atoms bond hexagonally, resembling a rolled-up sheet of chicken-wire fence.
The NEXAFS data also reveal that the boron nitride nanotubes have few defects and are highly crystalline - forming ordered arrangements - perhaps even more so than carbon nanotubes. This is an important property because many of the potential applications of boron nitride nanotubes, such as flat-panel displays, would require that they form crystalline patterns.
PUBLICATIONS
S. Banerjee, T. Hemraj-Benny, S. Sambasivan, D.A. Fischer, J.A. Misewich, and S.S. Wong, "NEXAFS Investigations of Order in Carbon Nanotube-Based Systems", invited contribution (George W. Flynn's Festschrift issue). J. Phys. Chem. B, 109(17), 8489-8495 (2005).
T. Hemraj-Benny, S. Banerjee, S. Sambasivan, D.A. Fischer, W. Han, J.A. Misewich, and S.S. Wong, "Investigating the Structure of Boron Nitride Nanotubes by Near-Edge X-ray Absorption Fine Structure (NEXAFS) Spectroscopy", Phys. Chem. Chem. Phys., 7(6), 1103-1106 (2005).
S. Banerjee, T. Hemraj-Benny, M. Balasubramanian, D.A. Fischer, J.A. Misewich, and S.S. Wong, "Surface Chemistry and Structure of Purified, Ozonized Multi-walled Carbon Nanotubes Probed by NEXAFS and Vibrational Spectroscopies", ChemPhysChem, 5, 1416-1422 (2004).
S. Banerjee†, T. Hemraj-Benny, M. Balasubramanian, D.A. Fischer, J.A. Misewich, and S.S. Wong, "Ozonized Single-walled Carbon Nanotubes Investigated using NEXAFS Spectroscopy", Chem. Commun., (7), 772-773 (2004).
Source: Brookhaven National Laboratory (by Laura Mgrdichian)