New insight into how rubber is made could improve tires, reduce air pollution
People have been making rubber products from elastic bands to tires for centuries, but a key step in this process has remained a mystery. In a report in the ACS journal Macromolecules, scientists have described this elusive part of rubber production that could have major implications for improving the material and its uses. Their findings, if used to improve tire performance, for example, could mean higher gas mileage for consumers and less air pollution.
Yuko Ikeda and colleagues note that a chemical process called vulcanization has been critical for the manufacturing of quality rubber since the second half of the 1800s. Chemists have improved the process, but progress has largely plateaued in recent years. If scientists could gain insight into the details of vulcanization, they could further tweak it to make even better rubber. Ikeda's team set out to uncover a key step in this process.
Using the latest analytical techniques, the researchers discovered a previously unknown structure that forms during vulcanization. The new observation could contribute to making the ubiquitous material even better. For the auto industry, resulting improvements in tire performance could translate to fuel savings and lower emissions, the researchers say.
More information: Dinuclear Bridging Bidentate Zinc/Stearate Complex in Sulfur Cross-Linking of Rubber, Macromolecules, 2015, 48 (3), pp 462–475. DOI: 10.1021/ma502063m
Abstract
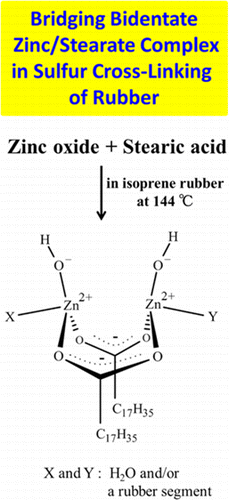
An essential structure of the intermediate generated from zinc oxide and stearic acid during sulfur cross-linking reaction of isoprene rubber is proposed using time-resolved zinc K-edge X-ray absorption fine structure and infrared spectroscopies in situ. The structure is dominantly a bridging bidentate zinc/stearate complex, the molar ratio of the zinc ion to stearate and the coordination number of which are unexpectedly 1:1 and 4, respectively. Combination with a density functional calculation for identifying the intermediate predominantly suggests that its most possible structure is (Zn2(μ-O2CC17H35)2)2+(OH–)2·XY, where X and Y are water and/or a rubber segment. This intermediate has been unknown despite the long history of rubber science and technology. The newly observed zinc/stearate complex may play a role to accelerate the sulfur cross-linking reaction of rubber like an enzyme because of the high Lewis activity of the zinc ion.
Provided by American Chemical Society


















