Airlock-like transport protein structure discovered
Sugars are an essential source of energy for microrganisms, animals and humans. They are produced by plants, which convert energy from sunlight into chemical energy in the form of sugars through photosynthesis.
These sugars are taken up into cells, no matter whether these are bacteria, yeast, human cells or plant cells, by proteins that create sugar-specific pores in the membrane that surrounds a cell. These transport proteins are thus essential in all organisms. It is not surprising that the transporters of humans and plants are very similar since they evolved from their bacterial ancestors.
Sugar transporters can also be a source of vulnerability for plants and animals alike. In plants they can be susceptible to takeover by pathogens, hijacking the source of the plant's food and energy. In animals, mutations in sugar transporters can lead to diseases, such as diabetes.
New work from a team led by the Stanford University School of Medicine's Liang Feng and including Carnegie's Wolf Frommer has for the first time elucidated the atomic structures of the prototype of the sugar transporters (termed "SWEET" transporters) in plants and humans. These are bacterial sugar transporters, called SemiSWEETs (because they are just half the size of the human and plant ones). Their work is published in Nature.
Until now, there was very limited information about the unique structures of these important transport proteins, which it turns out are different from all other known sugar transporters.
Discovering the structure of these proteins is important, as it is the key to unlocking the mechanism by which they work. And understanding their mechanism is crucial for figuring out what happens when these functions fail to work properly, because that knowledge can help in addressing the resulting diseases or growth problems in both plants and animals.
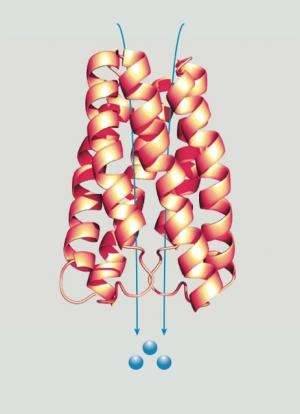
The research team performed a combination of structural and functional analyses of SemiSWEETs and SWEETs and was able to crystallize two examples in different states, demonstrating not only the protein's structure, but much about its functionality as well.
They found that the SemiSWEETs do not act as a sugar channel, or tunnel, which allow sugars to pass across the membrane. Rather they act like an airlock, moving the sugars in multiple stages, two of which can be observed in the crystal structures. The SemiSWEETs, among the smallest known transport proteins, assemble in pairs, thereby creating a structure that looks like their bigger plant and human SWEET homologs. This marks the SWEET family of proteins as drastically different from other sugar transport proteins.
"One of the most-exciting parts of this discovery is the speed with which we were able to move from discovering these novel sugar transporters, to determining their actual structure, to showing how they work," Frommer said. "Fantastic progress made possible by a collaboration with a structural biologist from Stanford University. Our findings highlight the potential practical applications of this information in improving crop yields as well as in addressing human diseases."
More information: Structures of bacterial homologues of SWEET transporters in two distinct conformations, Nature, DOI: 10.1038/nature13670
Journal information: Nature
Provided by Carnegie Institution for Science

















