Starving pancreatic cancer before it has a chance to feast
(Medical Xpress)—Pancreatic cancer's low survival rate gives researchers from The University of Kansas Cancer Center even more reason to find a way to prevent and treat the hard-to-detect cancer. Drs. Snigdha Banerjee, Ph.D., and Sushanta Banerjee, Ph.D., a husband and wife research team at the Kansas City VA Medical Center, are working towards cutting off the growth of pancreatic tumors before they can metastasize throughout the body. Their main target is angiogenesis—or creating new blood vessels from existing ones.
Pancreatic cancer and other cancers can only thrive, grow and spread if they have nutrients from blood, just like other tissues in our bodies. Cancer cells and tumors at first rely on nearby blood vessels to get what they need to survive, but, as tumors grow, they need to form new vessels. These vessels differ from those in regular tissue, Sushanta explained, which is part of the reason cancer can be so difficult to treat.
"Tumor blood vessels are different than regular blood vessels," said Sushanta. "Tumor vessels are sluggish and leaky, so when we try and treat cancer with drugs they can't easily reach the tumor because the vessels aren't strong."
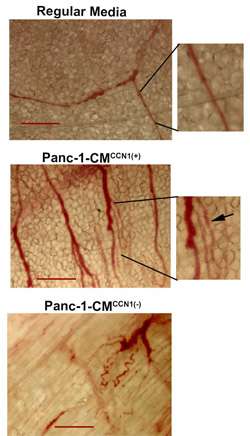
In previous research, the Banerjees discovered that a particular protein, CCN1, is overexpressed in pancreatic cancer, which leads to tumor formation. Knowing that this protein could be a key target in treating pancreatic cancer, they sought to understand exactly how it works. In a recent paper published in Scientific Reports (Nature publishing group), they explain how CCN1 signals tumor angiogenesis in pancreatic cancer.
CCN1 promotes new blood vessel growth in both normal and cancer tissues. But CCN1 does something extra when it's secreted by pancreatic tumor cells as opposed to regular cells. It both promotes angiogenesis within the tumor and increased the migration of endothelial cells, which form the inner lining of blood vessels. This migration process is key in the formation of the tumor vessels, according to the National Cancer Institute.
"The tumor cell secretes CCN1 into itself, which is a signal to start angiogenesis," said Snigdha. "So it seems that if you can target CCN1 with a drug, then the tumor will shrink and stop growing."
There is another piece necessary for the pancreatic cancer cell to start forming its own blood vessels - through their research, the Banerjees found that CCN1 is a crucial regulator of SHh, or the "sonic hedgehog" gene. They found that SHh activated by CCN1 also promotes blood vessel formation in the pancreatic cancer cells.
"Both of these components seem to be critical for angiogenesis," said Sushanta.
Understanding this process can lead to better targeted therapies for pancreatic cancer. When the Banerjees knocked down the CCN1 protein using shRNA, very few vessels and capillaries formed under mouse skin. This finding suggests that finding or creating a drug that silences CCN1 could slow down or even stop pancreatic cancer tumors from growing.
Pancreatic cancer is one of the most aggressive cancers. Nearly 40,000 people are estimated to die from pancreatic cancer in 2014 and there will be around 46,000 new cases diagnosed, according to the National Cancer Institute. Only 6.7 percent of those diagnosed with pancreatic cancer will go on to live at least five years.
"The next step would be to find an existing drug that can target the CCN1 protein, or create one," said Snigdha.













