New way to fight antibiotic-resistant bacteria: Target human cells instead
As more reports appear of a grim "post-antibiotic era" ushered in by the rise of drug-resistant bacteria, a new strategy for fighting infection is emerging that targets a patient's cells rather than those of the invading pathogens. The technique interferes with the way that the pathogens take over a patient's cells to cause infection. This approach, published in the journal ACS Chemical Biology, could help address the world's growing problem of antibiotic-resistant "super bugs."
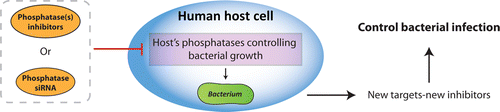
Huib Ovaa, Jacques Neefjes and colleagues explain that the problem of antibiotic-resistant bacteria poses a major public health threat. Health organizations have warned that unless alternatives to classic antibiotics are developed, even infections from minor scrapes could become deadly. Pharmaceutical companies are working on only a few new antibiotics, and they all take the same approach – attack the bacteria. But resistance is always a possibility. To get around this, researchers are now looking more closely at how bacteria co-opt the cells they invade for survival. These researchers previously reported that at least one set of host cell proteins, called kinases, can control bacterial growth. Ovaa and Neefjes' team decided to look at another class of proteins, called phosphatases, that act in the opposite way from kinases to see if inhibiting them would have a similar effect.
In lab tests, they identified phosphatases in human cells that are involved in bacterial survival. They also identified small molecules, or potential drugs, that could stop those phosphatases from working. Those molecules, which could form a new class of antibiotics, successfully stopped Salmonella, their test bacteria, from growing. Because this approach jams the host cell machinery rather than directly attacking the bacteria, the chances of bacteria developing resistance could be very low, say the researchers. They also say that the research shows that phosphatases, like kinases, could be general targets for drug development.
More information: "Integrating Chemical and Genetic Silencing Strategies To Identify Host Kinase-Phosphatase Inhibitor Networks That Control Bacterial Infection" ACS Chem. Biol., Article ASAP. DOI: 10.1021/cb400421a
Abstract
Every year three million people die as a result of bacterial infections, and this number may further increase due to resistance to current antibiotics. These antibiotics target almost all essential bacterial processes, leaving only a few new targets for manipulation. The host proteome has many more potential targets for manipulation in order to control bacterial infection, as exemplified by the observation that inhibiting the host kinase Akt supports the elimination of different intracellular bacteria including Salmonella and M. tuberculosis. If host kinases are involved in the control of bacterial infections, phosphatases could be as well. Here we present an integrated small interference RNA and small molecule screen to identify host phosphatase-inhibitor combinations that control bacterial infection. We define host phosphatases inhibiting intracellular growth of Salmonella and identify corresponding inhibitors for the dual specificity phosphatases DUSP11 and 27. Pathway analysis places many kinases and phosphatases controlling bacterial infection in an integrated pathway centered around Akt. This network controls host cell metabolism, survival, and growth and bacterial survival and reflect a natural host cell response to bacterial infection. Inhibiting two enzyme classes with opposite activities–kinases and phosphatases–may be a new strategy to overcome infections by antibiotic-resistant bacteria.
Journal information: ACS Chemical Biology
Provided by American Chemical Society