Implantable medical device for epilepsy
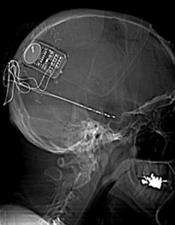
(Medical Xpress)—Physicians at the University of Rochester Medical Center (URMC) Strong Epilepsy Center were involved in the recent approval of a new treatment for epilepsy. The implantable medical device – called the Responsive Neurostimulator System (RNS) – monitors brain activity and can detect and counteract seizures.
URMC was one of only 28 sites in the country to conduct clinical trials of RNS, which was developed by the California-based company Neuropace. The research showed that the device decreases the number of monthly seizures by nearly 38 percent. URMC neurologists Michel Berg, M.D. and James Fessler, M.D., and neurosurgeon Web Pilcher, M.D., Ph.D. were involved in the study.
RNS was approved by the U.S. Food and Drug Administration (FDA) on November 14.
"This is the first FDA-approved brain implant for epilepsy that responds to the brain's activity," said Berg, an associate professor of Neurology. "For patients who are unable to control their seizures with medications or are not eligible for resective surgery, this device could provide an important treatment option."
An estimated three million Americans suffer from epilepsy. The condition, which can be triggered by a host of different factors, results in bursts of electrical activity in the brain caused when groups of neurons fire in an abnormal pattern. The resulting seizures can vary in length and severity.
While most individuals with the condition are able to manage their symptoms with one or more antiepileptic drugs, for a significant portion of people with epilepsy these medications are either not effective or result in intolerable side effects. Some of these patients are candidates for surgery which removes the region of the brain where the seizures originate.
However, when the seizures come from several different spots or are located in an area of the brain that serves an important function, such as language processing or motor control, resective surgery is not possible. For patients who have run out of traditional medical options, the RNS may be an effective way to control their seizures.
The RNS is an implantable device that is designed to suppress seizures before symptoms appear. The device functions in a manner akin to implantable cardiac defibrillators, which detect abnormal heart rhythms and then deliver electrical stimulation to correct them.
The RNS is surgically implanted under the scalp and connected to one or two leads – insulated wires with electrodes at the end – that are placed either on the surface of the brain or are guided into the brain in the area where the seizures are determined to originate. The RNS continuously monitors the patient's brain waves and when seizure activity is detected, the device instantly delivers a mild, brief electrical stimulation that suppresses the seizures.
"The RNS system is not only a technological breakthrough, but the device is an important new tool that could help patients control their seizures and, ultimately, improve their quality of life," said Fessler, the director of the Strong Epilepsy Center.