Getting down to the heart cells of the matter
(Phys.org) —Virginia Commonwealth University researchers have some new clues into what makes our tickers talk – on the molecular level.
In the heart, ion channels work together to control heart rate and rhythm. Unfortunately, channels that misbehave can cause an irregular heartbeat known as cardiac arrhythmia.
Gea-Ny Tseng, Ph.D., professor in the Department of Physiology and Biophysics in the VCU School of Medicine, and colleagues have been examining heart cells to determine the function and regulation of ion channels in the heart.
In a new study, published online and in the December print issue of the Journal of Biological Chemistry, Tseng and colleagues examined a "slow delayed rectifier," or IKs, channel. IKs requires two proteins associated with each other: KCNQ1, which is responsible for forming the ion passage way, and KCNE1, which is responsible for regulating the KCNQ1 channel function.
"When we are exercising or are under stress, IKs becomes larger," Tseng said.
"This is critical for stabilizing heart rate and rhythm. Our work identifies a novel mechanism for how this happens," she said.
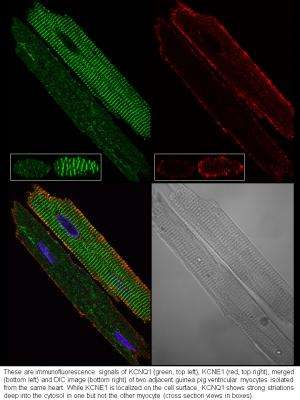
The team found that in resting heart cells, KCNQ1 is mainly inside the cells and KCNE1 is mainly on cell surface. In other words, according to Tseng, they are physically apart and cannot form functional IKs channels. However, when the team challenged heart cells by mimicking conditions in the heart during exercise or under stress, KCNQ1 travels to the cell surface, where it can associate with KCNE1 to form IKs channels.
"It has been assumed that KCNQ1 and KCNE1 are always associated in heart cells because this is the only way to have functional IKs channels. However, this assumption has not been tested experimentally until now," Tseng said.
"We think IKs may not be unique. Other channels may also travel to different locations, in response to cellular conditions, as a way to regulate their function or cell membrane potential."
Tseng said that patients that carry mutations in proteins KCNQ1 or KCNE1 suffer from congenital arrhythmia syndromes: long QT (LQT), short QT (SQT) or familial atrial fibrillation (fAF).
"The knowledge that KCNQ1 can dynamically change its location and regulate the IKs amplitude means that scientists may find methods to manipulate the trafficking pattern of KCNQ1," she said.
Although the research is still in its early stages, the hope is that the work may one day benefit patients with these mutations. According to Tseng, down the road, physicians may be able to use these methods to adjust the IKs amplitude as a therapeutic strategy – making IKs larger in LQT patients or make IKs smaller in SQT or AF patients.
To further the research, Tseng and colleagues are exploring ways to manipulate KCNQ1 trafficking in cells.
"We are particularly interested in finding ways to promote trafficking of KCNQ1 to the cell surface under resting conditions. We are working on a number of small molecules that can cause long-term potentiation of the IKs current amplitude," said Tseng. She said that preliminary experiments suggest that some of them can increase the cell surface KCNQ1 level.
Journal information: Journal of Biological Chemistry
Provided by Virginia Commonwealth University