Cell nucleus protein in brown fat cells governs daily control of body temperature
For nearly 300 years, investigators have known that body temperature follows a circadian, or 24-hour, rhythm, with a peak during the day and a low at night. The benefit of this control during evolution may have been to allow conservation of energy while sleeping because keeping body temperature above the surrounding temperature requires heat production from metabolic processes inside the body. But, it is also critical to be able to adapt to changes in ambient temperature, regardless of the time of day.
However, the mechanism responsible for coordinating daily body temperature rhythm and adaptability to environmental challenges is unknown. Now, the laboratory of Mitchell A. Lazar, MD, PhD, director of the Institute for Diabetes, Obesity, and Metabolism at the Perelman School of Medicine, University of Pennsylvania explains in Nature how body temperature rhythms are synchronized while maintaining the ability to adapt to changes in environmental temperature no matter the time of day or night.
"Food is plentiful in our present day society, and for most people there is unlikely to be an advantage of saving calories at night", says Lazar. "If this same mechanism exists in people, and we can target it safely, we could have a new way to combat obesity and its associated conditions such as diabetes and heart disease."
Lazar's team, including lead author Zachary Gerhart-Hines, Ph.D., found that the ability of mice to withstand a cold-temperature challenge was greater at 5:00 AM, when the mice are awake, compared to 5:00 PM when they are normally sleeping. This previously unrecognized circadian susceptibility to cold was found to be controlled by a protein called Rev-erb alpha, which is a molecular component of the body's biological clock mechanism.
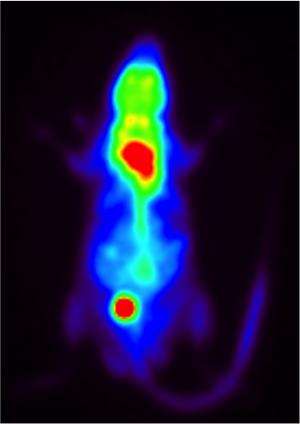
Indeed, mice engineered to lack the Rev-erb alpha protein had similar cold tolerance throughout the 24-hour day and also lost the physiological circadian rhythm of body temperature. The all-Penn team of authors represents a broad set expertise, including those who study brown fat physiology, radiology for PET scans of experimental mice, and basic physiologists who ruled out shivering as an explaining mechanism for the findings.
The Penn team concluded that Rev-erb alpha acts as a focal point for dialing body temperature up and down and is required for establishing and maintaining fundamental, body-temperature rhythm, while affording adaptability to respond to environmental demands of unpredictable changes in ambient temperatures.
Role of Brown Fat
Rev-erb alpha's effects were produced mainly by circadian regulation in brown adipose tissue, commonly called brown fat, the body's furnace. Brown fat cells, as opposed to white fat cells, make heat for the body and are thought to have evolved to help mammals cope with the cold. But, their role in generating warmth might also be applied to coping with obesity and diabetes. Brown fat cells are thought to counteract obesity by burning off excess energy stored in lipid, but white fat cells store energy. Indeed, brown fat cells contain many smaller droplets of lipids and the most mitochondria (containing pigmented cytochromes that bind iron) of any cell type, which make them brown. Mitochondria are the cells' energy factories in the form of the molecule ATP.
The mice that are exposed to cold fare dramatically better—their body temperature goes down less—early in the morning when Rev-erb alpha is barely present compared to the late afternoon, when Rev-erb alpha is abundant. Deletion of the gene Rev-erbα markedly improves cold tolerance at 5 PM, indicating that tolerance to cold is related to lower levels of the Rev-erb alpha protein.
More specifically, turning on the uncoupling protein 1 (UCP1) by cold temperatures is preceded by rapid downregulation of Rev-erbα in brown fat. Rev-erb alpha represses UCP1 in a brown fat cell-autonomous manner – the effect is direct in the brown fat, rather than operating through the nervous system. Brown fat UCP1 levels are high in mice without Rev-erbα even when ambient temperatures remain constant. Genetic loss of Rev-erbα also abolishes normal rhythms of body temperature and brown-fat activity.
The recent discovery of brown fat tissue in humans suggests that the same mechanism could underlie the daily fluctuations in body temperature in people. Although the lowering of body temperature may have had a survival advantage during evolution, by preserving energy stores when food was scarce, in modern times, lower body temperatures, and therefore lower metabolism and reduced burning of stored fats while sleeping, could contribute to the obesity problem. Knowledge of the mechanism that lowers body temperature at night may provide a way to burn calories while people are sleeping.
More information: The nuclear receptor Rev-erbα controls circadian thermogenic plasticity, DOI: 10.1038/nature12642