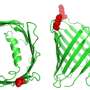
How disease mutations affect the Parkin protein
Researchers at the MRC Laboratory of Molecular Biology in the United Kingdom have determined the crystal structure of Parkin, a protein found in cells that when mutated can lead to a hereditary form of Parkinson's disease. The results, which are published in The EMBO Journal, define the position of many of the mutations linked to hereditary Parkinson's disease and explain how these alterations may affect the stability and function of the protein. The findings may in time reveal how the activity of Parkin is affected in patients with this rare but debilitating type of Parkinson's disease.
Parkinson's disease is a progressive neurodegenerative disease that affects more than seven million people worldwide. Most cases of the disease occur in older individuals and are sporadic (non-familial), but around 15% of patients develop symptoms early in life because of inherited mutations in a limited number of disease genes. Why Parkin mutations are especially detrimental in nerve cells is not fully understood, but previous research indicates that Parkin regulates the function of mitochondria, the organelles that generate energy in the cell. Some disease mutations in the PARKIN gene can be easily explained since they lead to loss or instability of the Parkin protein, but many others are more difficult to understand.
Around 50% of cases of familial recessive Parkinson's disease are caused by mutations in the PARKIN gene, which encodes a protein that belongs to the RBR ubiquitin ligase enzyme family. Enzymes in this family couple other proteins in the cell to a molecule called ubiquitin, a step that can alter the function or stability of these target proteins. To understand how Parkin and other RBR ubiquitin ligase enzymes achieve this, EMBO Young Investigator David Komander and his coworker Tobias Wauer crystallized a form of human Parkin and used X-ray diffraction patterns to determine how the Parkin protein chain folds into a three-dimensional structure. Their experiments revealed an in-built control mechanism for Parkin activity, which is lost in the presence of some of the mutations responsible for Parkinson's disease. Wauer and Komander pinpointed amino acids of Parkin with key functions in ubiquitin ligase activity that are sensitive to blocking by reagents previously characterized in their laboratory. "This sensitivity to inhibitors that were developed for a very different class of enzymes is particularly exciting," Komander remarked. "We could also show that these inhibitors affect related RBR ubiquitin ligases such as HOIP, which is important for inflammatory immune responses."
The crystal structure of Parkin is already revealing some of the secrets of this molecule, which under the right conditions can protect cells from the damage that arises during Parkinson's disease. "In time the structure may also allow development of other compounds that alter Parkin activity, which could serve as ways to limit the progression and impact of Parkinson's disease," concluded Komander.
More information: Structure of the human Parkin ligase domain in an autoinhibited state, Tobias Wauer and David Komander, doi: 10.1038/emboj.2013.125