Fanning the flames of tumor growth: Enzyme responsible for protecting chromosome ends stimulates tumorigenesis
Chromosomes are capped by long, repetitive DNA sequences called telomeres. These caps prevent genomic damage by insulating against the steady shortening of DNA ends that naturally accompanies replication. Once mature, cells generally stop producing the telomere-building enzyme telomerase and stop dividing when these caps have shortened to a critical length. However, many cancer cells get around this restriction by restoring telomerase production, allowing uncontrolled growth.
Several studies have indicated that telomerase performs functions other than chromosome capping. Research from Vinay Tergaonkar's team at the A*STAR Institute of Molecular and Cell Biology in Singapore has revealed how NF-κB—another protein abnormally activated in many cancers—not only stimulates release of signals that promote an inflammatory response to help beat back infectious threats, but can also establish physiological conditions that favor cancerous growth if left unchecked. "Chronic inflammation and telomerase reactivation are hallmarks of most human cancers," says Tergaonkar, "but the mechanism of how enhanced NF-κB and telomerase activities are each sustained in cancers is unknown."
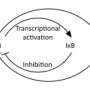
Their experiments revealed a surprisingly close relationship between these processes. Boosting telomerase activity in cultured human cancer cells enhanced tumorigenesis, but these effects could be countered by forcing cells to produce lower levels of NF-κB. The researchers subsequently demonstrated that telomerase directly enhances NF-κB activity (see image), and found that genetically modified mice lacking telomerase showed a greatly reduced inflammatory response following exposure to bacterial toxins.
Similar effects were apparent when Tergaonkar's team compared NF-κB activity in telomerase-producing and deficient cells. Since both cell lines exhibited equivalent telomere lengths, these results favor a telomere-independent mode of action. The researchers demonstrated that telomerase binds directly to numerous NF-κB target genes, and actually strengthens NF-κB's association with several of these genomic sites. For example, telomerase and NF-κB collaboratively stimulate production of the major inflammatory signal interleukin-6 (IL-6). Tergaonkar and co-workers also showed that a chemical inhibitor of telomerase dramatically reduced IL-6 production in a wide variety of leukemia cell lines.
Perhaps most importantly, the gene encoding telomerase is itself a target of NF-κB, creating a 'vicious circle' of signaling. "The two pathways fuel each other's activities," says Tergaonkar. "These findings hence provide a unifying mechanism for the sustained inflammation seen in a vast majority of cancers, and identify telomerase as a novel regulator of inflammation." In future efforts, his team will explore how telomerase exerts its gene regulatory effects and seek out potential partner molecules that assist in this process.
More information: Ghosh, A., Saginc, G., Leow, S. C., Khattar, E., Shin, E. M. et al. Telomerase directly regulates NF-κB-dependent transcription. Nature Cell Biology 14, 1270–1281 (2012). www.nature.com/ncb/journal/v14 … 12/full/ncb2621.html