Neighboring immune-system genes: Maintaining independence
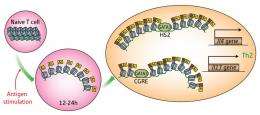
As part of the immune response to foreign antigens, naïve T cells mature into different types of helper T cells. TH1 cells and TH17 cells, for example, secrete a subset of signaling factors known as cytokines that promote inflammatory responses to viral infections, while TH2 cells secrete cytokines that promote antibody secretion by B cells and drive allergic reactions.
The GATA-3 protein is known as a 'master switch' for TH2 differentiation, stimulating production of cytokines such as interleukin (IL)-4 and IL-13, but new findings from a team led by Masato Kubo at the RIKEN Center for Allergy and Immunology in Yokohama have revealed an unexpected degree of complexity in this activation process.
“The idea that genes encoding TH2 cytokines are coordinately regulated … has been widely accepted,” says Kubo. Many of these genes are situated in the same chromosomal neighborhood, and some scientists believe that the chromosome physically loops so that DNA-bound GATA can regulate multiple sites simultaneously. However, Kubo and colleagues found that GATA appears to independently bind multiple, distinct sites that each confer regulatory control over individual TH2-associated genes.
One of these sites, HS2, specifically governs IL-4 expression, and GATA binding at this site induces chemical modification of the DNA segment containing the Il4 gene, leading to increased cytokine production. Naïve T cells from mice lacking this chromosomal region give rise to TH2 cells that generate normal levels of most cytokines, but fail to produce IL-4; these animals also show fundamental defects in their allergic response.
In parallel, the researchers identified a second GATA-binding site, CGRE, which specifically regulates production of IL-13. Like HS2, GATA interaction with this site is associated with targeted chemical modification of a nearby stretch of DNA containing the Il13 gene, and disruption of CGRE essentially eliminates production of this cytokine while leaving IL-4 production unaffected. “These results came as a surprise,” says Kubo. “They indicate that the independent recruitment of GATA-3 to locus-specific regulatory elements controls the expression status of individual genes encoding TH2 cytokines.” These findings also parallel previous data suggesting that GATA coordinates expression of IL-5, another TH2 cytokine, independently of IL-13.
Other types of immune cells also secrete TH2 cytokines, and Kubo and colleagues now hope to determine whether their findings represent a broadly used mechanism for regulating production of these cytokines. “Our next priority will be exploring the relative contribution of these discrete elements to transcriptional regulation of IL-4 and IL-13 among these cell types,” he says.
The corresponding author for this highlight is based at the Laboratory for Signal Network, RIKEN Research Center for Allergy and Immunology.
More information: Tanaka, S., et al. The enhancer HS2 critically regulates GATA-3-mediated Il4 transcription in TH2 cells. Nature Immunology 12, 77–85 (2011).















