Researchers study how ice melts in contact with soil
A team of scientists from the Max Planck Institute for Metals Research in Stuttgart (Germany) and the ESRF in France has studied how ice starts to melt at temperatures as low as - 17ºC. This can occur when ice is in contact with SiO2, a material commonly found in soil. Below the melting temperature of ice, a layer much denser than ‘regular’ water forms between the ice and the SiO2. The researchers were able to observe such changes occurring thanks to the powerful X-rays at the ESRF. These results may help to explain natural phenomena such as how glaciers slide or the stability of permafrost. The results of this study have just been published in Physical Review Letters.
Water is well known for its strange properties such as its expansion during the transition from liquid to solid. At school, students learn that the density of water is 1 g/cm3. This is somewhat of an over simplification, however, due to the complex behaviour and relationships between water molecules. In reality water is thought to exhibit density fluctuations between a high-density liquid (HDL) and a low-density liquid (LDL) on very short time scales.
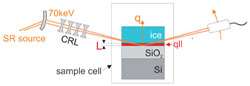
The team of scientists working at the ESRF discovered an odd water layer thanks to the high-energy X-ray microbeams of the synchrotron. They started by attaching crystalline ice onto silicon dioxide. The 24 mm long sample was kept in a specially-designed chamber in which the temperature of the sample could be stabilised and accurately controlled. As the X-rays penetrated the sample to the interface between water and silicon dioxide, the researchers started heating it from -25ºC to 0ºC. By the time the structure reached the melting point, the sample already contained a 5 nm layer of water. This water was found to be 20% more compact than normal water, having a density of 1.2 g/cm3.
These results represent a step forward in understanding the behaviour of ice. They may help explain natural processes such as the movement of glaciers. The motion of glaciers can mainly be explained by the internal deformation induced by gravity, (being relatively slow at around only 10 m/year). Another process which is thought to contribute to this movement is basal sliding. Basal sliding can occur ten times faster when the base of the ice is near the melting point and some water is present to enhance glacier movement. Nevertheless, observing the results of the experiment performed at the ESRF, this pre-melting phase at a lower temperature than the melting point could support a basal sliding theory.
The ramifications of this study are not only confined to glaciers. Permafrost is another example where a lower melting temperature could further our understanding. Permafrost describes rock or soil composite structures that remain below 0ºC for two or more years and often contain more than 30% ice. Permafrost areas cover large inhabited regions, yet the interfacial-melting phenomenon is not well understood. The results confirmed by the team of researchers at the ESRF could be important for civil engineering projects within these regions.
The results of this experiment open the way for new research: “we will study how the ice behaves in contact with different solids instead of silicon dioxide”, explains Veijo Honkimäki, one of the authors of the paper.
Source of this news release: EUROPEAN SYNCHROTRON RADIATION FACILITY
Publication: S. Engemann et al. Interfacial melting of ice in contact with SiO2, PHYSICAL REVIEW LETTERS, 92, 205701 (2004).