New fluorescence technique opens window to protein complexes in living cells
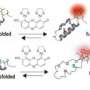
Fluorescent microscopy makes use of molecules, such as green fluorescent protein (GFP), that emit colored light when illuminated with light of a specific wavelength. Molecules like GFP can be used to label proteins of interest and can reveal information about the relationships of molecules within cells. Fluorescence polarization, also known as anisotropy, is specific parameter of fluorescence that can provide additional information about the properties of individual molecules.
Fluorescence anisotropy has been used to study isolated molecules. Now, a recent study describes a novel fluorescence anisotropy method that studies the dynamics of large protein complexes in real time in live cells. The research, published by Cell Press on September 21 in Biophysical Journal, is significant as the powerful new approach could be useful for studying many different types of intracellular protein complexes.
Dr. Sanford M. Simon, from The Rockefeller University, led a research group who developed and used the new approach to study individual protein domains within a complex structure called the nuclear pore complex (NPC). The NPC is a large assembly of proteins that cross the double membrane that surrounds the nucleus of a cell. The NPC regulates the transport of substances into and out of the nucleus, which houses most of the cell's genetic material. "Recent structural studies have revealed some atomic detail of NPC architecture, but the dynamics and molecular mechanism of this central machinery of life are largely unknown," says Dr. Simon.
By fluorescently tagging specific domains of individual nucleoporins, the protein building blocks of the NPC, the researchers found both rigid and flexible regions. "We found that fluorescence anisotropy measurements of GFP-tagged nucleoporins resolved the state of order or disorder of protein domains in live budding yeast and there were differences between different domains of the same protein," explains Dr. Simon. The technique can also reveal information about the physical arrangement of individual protein domains within the NPC.
This use of fluorescence anisotropy may also be useful for studying different types of proteins. "This fluorescence anisotropy technique can be generalized and applied to discern order and disorder in other macromolecular complexes, providing they have symmetry or are organized relative to a greater structure," concludes Dr. Simon. "Importantly, anisotropy imaging can be accomplished in live cells, yielding a new and complementary link between structure, dynamics, and the actual function of protein complexes in their native environment," adds Dr. David W. Piston, from Vanderbilt University, who wrote a "New and Notable" article which accompanies the paper.
More information: Mattheyses et al.: "Fluorescence anisotropy reveals order and disorder of protein domains in the nuclear pore complex." The Biophysical Journal, September 22, 2010. www.biophysics.org/
Provided by Cell Press