Regulating prions naturally
Prions can form in fungi, but there is also a prion antagonist that regulates the system. A glimpse into the unusual properties of proteins.
For quite some time prions were practically on everyone's lips. Images of BSE-infected cattle flashed around the globe. Hundreds of thousands of cows died. People were afraid of contracting the deadly disease from contaminated meat. The trigger for the disease are prions - pathogenic proteins that after a change in their structure aggregated into what are known as amyloids, causing them to mutate into aggressive pathogens.
There were fears of an epidemic, which fortunately did not eventuate. Prions have since disappeared from the newspaper columns, but not from the research laboratories. Scientists continue to investigate these remarkable pathogens and they’re discovering more and more about the functions that the aggregrating proteins have in nature.
Basic immune system
As a paper by Roland Riek, Professor of Physical Chemistry at ETH, published recently in the journal Molecular Cell reveals, prions are part of a rudimentary immune system in fungi, and are involved in a cellular self-recognition process: cells of different fungal strains use prions to prevent individual fungi from exchanging genetic viral information with one another.
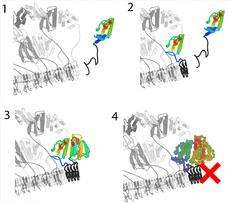
The fungus Podospora anserina, for instance, has two very similar proteins that behave very differently: HET-s and HET-S. The first of these, the HET-s protein, is able to easily change its conformation and convert into a prion that can infect other similar fungi. The prions stack themselves up like Lego bricks into filamentous structures, and by recruiting further HET-s molecules the Lego tower can grow longer. The HET-s prion continuously increases in size, but is not toxic.
The protein HET-S is different in that although it is almost identical to HET-s except for a small number of amino acid residues, it cannot become a prion itself. Instead, HET-S is a regulator of the HET-s prion, as the ETH researchers have been able to demonstrate. HET-S can connect to the HET-s prion, inhibiting its infectiousness. The ETH researchers have discovered that just one one-hundredth of the prion stopper HET-S is sufficient to slow down the aggregation of HET-s into infectious amyloids.
Cell death protects against infection
If two different Podospora anserina fungi, one with HET-s prions and the other with HET-S proteins, fuse together, the HET-S prevents the prion chain reaction from continuing. In addition, the resulting co-aggregate of the HET-S protein and the HET-s prion that forms in the fusion cell is toxic, resulting in a localised and controlled dying off of the cells at the point of contact of the fungi. The HET-s prion of a HET-s fungus is therefore a marker that prevents a genetically distinct HET-S fungus from combining with it. “This is the most basic form of an immune system”, says Roland Riek.
His research group has clarified in meticulous detail not only the functions, but also the atomic structure of the two molecules. In the process, they have identified which sections of the proteins are responsible for the aforementioned processes.
Combating amyloid diseases
The fungus and its proteins HET-s and HET-S actually only represent a model system for the mammalian prions. Nevertheless, this system presents a possible approach to combating the diseases associated with amyloid formation, including Alzheimer’s, Parkinson’s and Type II diabetes, among others.
“Due to the association of amyloids with diseases, it is difficult to imagine that amyloids such as the HET-s prion could also have a beneficial role”, says Riek. However, it is not only in fungi that protein amyloids are of value. Last year, Riek and his colleagues demonstrated that the human body aggregates certain hormones, which are also proteins, into amyloids and stores them in so-called secretory granules; the body is then able to quickly mobilise these substances as needed. In cases of physical stress, for example, the body releases endorphins relatively quickly, a rate which could not be produced by de novo protein synthesis. Here, nature is relying on the special aggregation properties of protein amyloids - this time to humankind’s advantage. Thus, prions and amyloids also have beneficial functions. “We have been able to show that the aggregation of proteins occurs naturally and that it does not necessarily result in dangerous diseases”, emphasises Riek.
More information:
-- Greenwald J et al. The Mechanism of Prion Inhibition by HET-S. Molecular Cell, Volume 38, Issue 6, 889-899, 25 June 2010. DOI:10.1016/j.molcel.2010.05.019
-- Maji SK et al. Functional Amyloids As Natural Storage of Peptide Hormones in Pituitary Secretory Granules. Science 17 July 2009: Vol. 325. no. 5938, pp. 328 - 332. DOI:10.1126/science.1173155
Provided by ETH Zurich















