Scientists watch chemical bond break using molecule's electrons
Scientists at the National Research Council of Canada (NRC) and the University of Ottawa (uOttawa) enjoyed a bird's eye view of a chemical bond as it breaks.
The making and breaking of chemical bonds underlie the biochemical processes of life itself. A greater understanding of the quantum processes that lead to chemical reactions may lead to new strategies in the design and control of molecules — ultimately leading to scientific breakthroughs in health care and diagnostic medicine, quantum computing, nanotechnology, environmental science and energy.
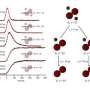
The NRC-uOttawa team, led by Dr. David Villeneuve, achieved their feat using a technique developed several years ago at NRC in which an image was obtained of a single electron orbiting a molecule. In the current experiment, which is reported in the July 29th edition of Nature, scientists injected bromine gas into a vacuum chamber. There, an ultra brief ultraviolet light pulse caused the bromine molecules to separate into their individual atoms (a bromine molecule is composed of two bromine atoms).
A few femtoseconds later, an intense infrared laser pulse caused the molecule to emit an attosecond-duration X-ray burst that contained a snapshot of the atom's position as the molecule fell apart and revealed how the electrons rearranged themselves.
"Due to the strange laws of quantum physics," Dr. Villeneuve explains, "a molecule that is broken apart by an ultraviolet laser pulse is at the same time unaffected by the pulse, a paradox, much like Schrödinger's Cat is both dead and alive."
The interference of the x-rays emitted by the two quantum states of the molecule was used to find the location of the atoms and to watch over a period of only 200 femtoseconds as it progressed from being a molecule to being two separate atoms. The experiment reached a precision below 500 zeptoseconds in clocking the emitted x-ray bursts. "It is exciting to see the quantum transformation as it goes from being a molecule, in which electrons are shared, to individual atoms, says Villeneuve.
According to Professor Paul Corkum, co-author and a pioneer in attosecond physics, "In real life we are most sensitive to motion if there is a fixed background for reference. We have shown that it is the same in the molecular world. Unreacting molecules - usually a nuisance in an experiment - can also form a reference. Against this fixed background we become so sensitive to motion that we can see just few dissociating molecules. The experiment is another important step towards the dream of filming chemical reactions."
The research was conducted at JASLab, the Joint Attosecond Science Laboratory, a shared laser facility between the National Research Council of Canada and the University of Ottawa, with the participation of the Technical University of Vienna. JASLab is one of the top laboratories in the world conducting research on the attosecond timescale.
Provided by National Research Council of Canada