New genetic analysis reveals principles of phenotypic expression
The Human Genome Project, along with numerous parallel efforts to solve the DNA sequences of hundreds of animal, plant, fungal, and microbe genomes in the last few decades, has produced enormous amounts of genetic data with which researchers are struggling to keep pace. Knowing gene sequences, after all, may not directly reveal what roles that genes play in the actual manifestation of physical traits (or phenotypes) of an organism -- including their roles in human diseases. To help navigate the new genomic landscape, researchers are developing experimental approaches and analysis tools to help prioritize and organize complex genetic information with respect to phenotypic effects.
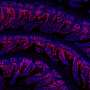
In the journal Chaos scientists at the University of Alabama at Birmingham report powerful new techniques for studying the phenotypes related to genetic differences in the budding yeast, Saccharomyces cerevisiae. The researchers took yeast cultures from an extensive library of approximately 5,000 mutated strains and subjected them to hydroxyurea -- an anti-cancer drug with known effects on the cell cycle.
Using a method called quantitative high-throughput cellular phenotyping (Q-HTCP), the researchers analyzed growth curves for tens of thousands of individual cultures, "focused on finding all of the genes that modulate the cellular effects of the drug," says study co-author John Hartman, an assistant professor of genetics. The researchers then selected the 300 "most 'hydroxyurea-interactive' genes" and further classified the genes by testing their influence on cell growth after treatment with drugs acting by different mechanisms.
To integrate the results from such experiments, the researchers developed a new data mining approach called Recursive Expectation-Maximization Clustering (REMc). The approach, Hartman says, "has advantages over prior methods with respect to defining cluster number and quantifying cluster quality," which augments biological discovery.
The technique, Hartman adds, "offers a new way for trying to understand how genetic variation -- such as that related to human disease -- is alternatively buffered or expressed." Understanding phenotypic expression at a systems level, he says, would help create a new field of medicine, dubbed "phenomics."
More information: The article, "Recursive Expectation-Maximization clustering (REMc): A method for identifying buffering mechanisms composed of phenomic modules" by Jingyu Guo et al will appear in Chaos: An Interdisciplinary Journal of Nonlinear Science. See: chaos.aip.org/
Provided by American Institute of Physics