Researchers Discover How to Move Protons, Improve Hydrogen Fuel Cell Technology
(PhysOrg.com) -- In a breakthrough that should help to solve one of the biggest problems holding back development of affordable fuel cells, a team of University of Massachusetts Amherst scientists has discovered a way to improve proton conductivity under very low humidity conditions, where few materials perform well at present.
The current generation of hydrogen fuel cells produces electricity by first splitting hydrogen into protons and electrons, where electrons go through the fuel cell electrical circuit while protons have to pass through a synthetic membrane. On the other side of the circuit, the protons and electrons combine with oxygen to produce water. This chemical reaction produces electrical energy and because the byproduct is water, the technology is environmentally friendly.
One of the basic problems in this clean energy technology is that these fuel cells prefer operating temperatures well above the boiling point of water, that is, they like low humidity. However, there are few efficient materials that conduct protons under such conditions. Now chemist Sankaran “Thai” Thayumanavan, director of the National Science Foundation’s Fueling the Future Center for Chemical Innovation at UMass Amherst, in collaboration with polymer scientist Ryan Hayward and physicist Mark Tuominen and their graduate students, has developed a materials design principle capable of addressing this need. Their findings are reported in the current issue of Nature Chemistry.
The UMass Amherst researchers have shown that materials that assemble into a structure that provides nanometer-size channels are capable of efficiently transporting charge. These channels provide an excellent conduit for moving protons from one side of the membrane material to another, which is critical for efficient fuel cell operation. Their discovery will help to design materials that could lead to commercial development of longer-lasting membranes that stay chemically and mechanically stable much longer than the current type, while maintaining efficiencies at the desired operating temperature.
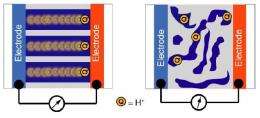
Thayumanavan says this is an “incredibly exciting development” relying on a polymer nanostructure that achieves superior results in a completely non-intuitive way, by combining both conducting and non-conducting domains in the membrane. As he explains this special assembly, “One would think that using a 100 percent conducting domain between the electrodes would be most efficient for proton conduction, but that’s not the case. What we’ve found is that by combining two opposing domains, conducting and nonconducting, in the membrane’s nanostructured assembly, we could improve its conductivity performance.”
This solution was inspired by nature, he adds. “We took a cue from these naturally occurring proteins which can transport proton groups inside our bodies over distances of a few nanometers at extremely fast speeds without using water. We hypothesized that just as in these proteins, certain shapes or combinations of block copolymers that combine some conducting and some nonconducting nanostructures might conduct protons better than a uniform matrix.”
This nonintuitive approach paid off, Thayumanavan reports, confirming that a 100-percent conducting domain is not as efficient as their mixed-property domain. “It turns out that a nanoscale assembly packed with domains that are mutually not attractive to each other, and are not usually found together, will create enhanced conductivity by about 1,000 times.” He and colleagues have now tried the new membrane design in four different sets of polymers with subtle variations and “it’s not a fluke.”
Provided by University of Massachusetts Amherst















