Scorpion venom provides clues to cause, treatment of pancreatitis
A Brazilian scorpion has provided researchers at North Carolina State University and East Carolina University insight into venom's effects on the ability of certain cells to release critical components. The findings may prove useful in understanding diseases like pancreatitis or in targeted drug delivery.
A common result of scorpion stings, pancreatitis is an inflammation of the pancreas. ECU microbiologist Dr. Paul Fletcher believed that scorpion venom might be used as a way to discover how pancreatitis occurs - to see which cellular processes are affected at the onset of the disease. Fletcher pinpointed a protein production system found in the pancreas that seemed to be targeted by the venom of the Brazilian scorpion Tityus serrulatus and then contacted NC State physicist Dr. Keith Weninger, who had studied that particular protein system.
"This particular protein system has special emphasis at two places in the body - the pancreas and the nervous system," Weninger says. "In the pancreas, it is involved in the release of proteins through the membrane of a cell." The pancreas specializes in releasing two kinds of proteins using separate cells: digestive enzymes that go into the small intestine and insulin and its relatives that go into the bloodstream, yet this same release mechanism is important in all of our cells for many processes.
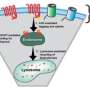
Cells move components in and out through a process called vesicle fusion. The vesicle is a tiny, bubble-like chamber inside the cell that contains the substance to be moved, stored and released - in this case, proteins like enzymes or hormones. The vesicle is moved through the cell and attaches to the exterior membrane, where the vesicle acts like an airlock in a spaceship, allowing the cell membrane to open and release the proteins without disturbing the rest of the cell's contents. The proteins that aid in this process are known as Vesicle Associated Membrane Proteins, or VAMPs.
Weninger provided Fletcher with two different VAMP proteins found in the pancreas, VAMP2 and VAMP8. They were engineered to remove the membrane attachments so they could be more easily used for experiments outside cells and tissues. Fletcher's team demonstrated that the scorpion venom attacked the VAMP proteins, cutting them in one place and eliminating the vesicle's ability to transport its protein cargo out of the cell.
The results were published in the March 5 issue of the Journal of Biological Chemistry.
"We found that a particular enzyme in the scorpion's venom removes a peptide, or small protein, that allows the vesicle to fuse with the cell membrane," Fletcher says. "If you remove a pancreatic cell's ability to absorb or release components, you end up with pancreatitis."
"Viruses often exploit the same mechanism of vesicle fusion, but in reverse, in order to invade cells and replicate," Weninger adds. "This work furthers our understanding of a basic cellular process and may lead to treatments for viruses and advances in treatments like chemotherapy, by allowing targeted drug delivery only to cancer cells."
More information: "Vesicle-associated Membrane Protein (VAMP) Cleavage by a New Metalloprotease from the Brazilian Scorpion Tityus serrulatus", Paul L. Fletcher, et al., March 5, 2010, in Journal of Biological Chemistry; VOL. 285, NO. 10, pp. 7405.
Provided by North Carolina State University