Helium rain on Jupiter explains lack of neon in atmosphere
(PhysOrg.com) -- On Earth, helium is a gas used to float balloons, as in the movie "Up." In the interior of Jupiter, however, conditions are so strange that, according to predictions by University of California, Berkeley, scientists, helium condenses into droplets and falls like rain.
Helium rain was earlier proposed to explain the excessive brightness of Saturn, a gas giant like Jupiter, but one-third the mass.
On Jupiter, however, UC Berkeley scientists claim that helium rain is the best way to explain the scarcity of neon in the outer layers of the planet, the solar system's largest. Neon dissolves in the helium raindrops and falls towards the deeper interior where it re-dissolves, depleting the upper layers of both elements, consistent with observations.
"Helium condenses initially as a mist in the upper layer, like a cloud, and as the droplets get larger, they fall toward the deeper interior," said UC Berkeley post-doctoral fellow Hugh Wilson, co-author of a report appearing this week in the journal Physical Review Letters. "Neon dissolves in the helium and falls with it. So our study links the observed missing neon in the atmosphere to another proposed process, helium rain."
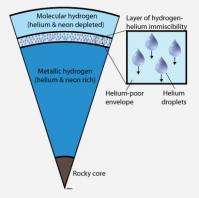
Wilson's co-author, Burkhard Militzer, UC Berkeley assistant professor of earth and planetary science and of astronomy, noted that "rain" - the water droplets that fall on Earth - is an imperfect analogy to what happens in Jupiter's atmosphere. The helium droplets form about 10,000 to 13,000 kilometers (6,000-8,000 miles) below the tops of Jupiter's hydrogen clouds, under pressures and temperatures so high that "you can't tell if hydrogen and helium are a gas or a liquid," he said. They're all fluids, so the rain is really droplets of fluid helium mixed with neon falling through a fluid of metallic hydrogen.
The researchers' prediction will help refine models of Jupiter's interior and the interiors of other planets, according to Wilson. Modeling planetary interiors has become a hot research area since the discovery of hundreds of extrasolar planets living in extreme environments around other stars. The study will also be relevant for NASA’s Juno mission to Jupiter, which is scheduled to be launched next year.
Militzer and Wilson are among the modelers, using "density functional theory" to predict the properties of Jupiter's interior, specifically what happens to the dominant constituents - hydrogen and helium - as temperatures and pressures increase toward the center of the planet. These conditions are yet too extreme to be reproduced in the laboratory. Even experiments in diamond-anvil cells can only produce pressures at the Earth's core. In 2008, Militzer's computer simulations led to the conclusion that Jupiter's rocky core is surrounded by a thick layer of methane, water and ammonia ices that make it twice as large as earlier predictions.
The two modelers embarked on their current research because of a discovery by the Galileo probe that descended through Jupiter's atmosphere in 1995 and sent back measurements of temperature, pressure and elemental abundances until it was crushed under the weight of the atmosphere. All elements seemed to be as slightly enriched compared to the abundance on the sun - which is assumed to be similar to the elemental abundances 4.56 billion years ago when the solar system formed - except for helium and neon. Neon stood out because it was one-tenth as abundant as it is in the sun.
Their simulations showed that the only way neon could be removed from the upper atmosphere is to have it fall out with helium, since neon and helium mix easily, like alcohol and water. Militzer and Wilson's calculations suggest that at about 10,000 to 13,000 kilometers into the planet, where the temperature about 5,000 degrees Celsius and the pressure is 1 to 2 million times the atmospheric pressure on Earth, hydrogen turns into a conductive metal. Helium, not yet a metal, does not mix with metallic hydrogen, so it forms drops, like drops of oil in water.
This provided an explanation for the removal of neon from the upper atmosphere.
"As the helium and neon fall deeper into the planet, the remaining hydrogen-rich envelope is slowly depleted of both neon and helium," Militzer said. "The measured concentrations of both elements agree quantitatively with our calculations."
Saturn's helium rain was predicted because of a different observation: Saturn is warmer than it should be, based on its age and predicted rate of cooling. The falling rain releases heat that accounts for the difference.
Jupiter's temperature is in accord with models of its cooling rate and its age, and needed no hypothesis of helium rain until the discovery of neon depletion in the atmosphere. Interestingly, theoretician David Stevenson of the California Institute of Technology (Caltech) predicted neon depletion on Jupiter prior to the Galileo probe's measurements, but never published a reason for his guess.
More information: Sequestration of Noble Gases in Giant Planet Interiors, Hugh F. Wilson and Burkhard Militzer, Phys. Rev. Lett. 104, 121101 (2010) - Published March 22, 2010, Download PDF (free)
Provided by University of California - Berkeley