March 11, 2010 report
New charging method could greatly reduce battery recharge time
(PhysOrg.com) -- Part of the headache of having to constantly recharge batteries is not just how often they need to be charged, but also the time it takes to charge them. In a new study, researchers have proposed a charging method that could greatly reduce the charging time of lithium-ion batteries, which are used in everything from electronic devices to electric vehicles. The new method uses an additional oscillating electric field (besides the charging field) that should be capable of charging a lithium-ion battery in a fraction of the time compared with traditional methods.
Researchers Ibrahim Abou Hamad from Mississippi State University and coauthors have developed the new charging method thanks to revolutionary developments in molecular dynamics simulations. In their study, the researchers simulated the lithium-ion battery-charging process by simulating the intercalation (i.e. “insertion”) of lithium ions into the battery’s graphite anode. Although intercalation is just one part of the charging process (along with diffusion), it dominates the charging time.
In the charging process, lithium ions first diffuse within the battery’s electrolyte until they reach the graphite anode. At this interface, ions must overcome an energy barrier in order to be intercalated into the anode.
In their simulations, Hamad and his team found that an additional oscillating electric field can lower this energy barrier, enabling lithium ions to intercalate more quickly into the anode. The oscillating field also increases the diffusion rate, which helps further reduce the overall charging time, albeit to a lesser extent.
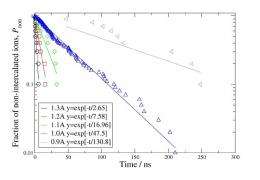
Specifically, when the scientists applied an oscillating square-wave field with a frequency of 25 GHz and an amplitude of 5 kCal/mol to the graphite sheets in the anode, the lithium ions intercalated into the graphite sheets within an average time of about 50 nanoseconds. By changing the amplitude of the oscillating wave, the researchers found that they could further improve charging time by lowering the energy barrier and speeding up intercalation. Their simulations showed that the dependence of the intercalation time on the amplitude is exponential, meaning that a small increase in amplitude leads to a large increase the intercalation speed, which offers the potential for very fast charging times.
In the future, the researchers plan to further investigate the new method, including analyzing how changing the frequency of the oscillating field effects the charging time. They noted that the new method might provide an increase in battery power densities, as well.
More information:
Ibrahim Abou Hamad, M. A. Novotny, D. Wipf, and P. A. Rikvold. “A new battery-charging method suggested by molecular dynamics simulations.” Available at arxiv.org. Doi: 10.1039/b920970k.
via: Technology Review
© 2010 PhysOrg.com