The mode of action of certain toxins that accumulate in seafood
Toxins released by certain microalgae can contaminate fish and shellfish which then become toxic to humans. French researchers from CNRS and CEA have, for the first time, identified the mechanisms of action of two of these toxins.
They have shown how and why they cause neurological symptoms. These findings could provide a basis for the development of new tests to screen for these toxins. This work was published online this week on the website of the journal PNAS.
Marine biotoxins are produced naturally by several species of single-cell algae. They can accumulate in the flesh of fish and shellfish and are then referred to as phycotoxins. In humans, the consumption of shellfish contaminated by these substances can cause diarrheal, paralytic, neurological and other symptoms. Phycotoxins can spread rapidly throughout the world, notably via the emptying of ballast tanks by merchant ships. As early as 1991, shellfish contamination was observed in Canada, then along the coasts of Norway, Spain and Tunisia. In 2005, contaminated oysters were detected in the Arcachon Basin on the west coast of France, which led the health authorities to impose a temporary ban on their sale.
A Franco-American collaborative project involving two joint CNRS/University laboratories in Marseilles, a CNRS intra-mural laboratory, a CEA laboratory in Gif-sur-Yvette and an American laboratory at the University of California has studied the functioning of two types of phycotoxins, a spirolide and a gymnodimine. These are "rapid-acting" neurotoxins; their injection in laboratory mice causes severe neurological symptoms that have a fatal outcome within a few minutes. The researchers were able to characterize the target of these toxins: they attack a receptor that is essential in living beings, the nicotinic acetylcholine receptor (nRACh), a channel receptor situated on the membrane of muscle or nerve cells that allows the passage of small ionized molecules into and out of the cell. nRACh plays a crucial role in neuromuscular and neuronal transmission. More precisely, these toxins act by rapidly and almost irreversibly blocking the channel receptor function of nRAChs. This inhibition then causes muscle and/or cerebral dysfunctions, reminiscent of those observed during certain muscle diseases or cognitive disorders.
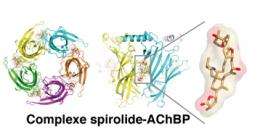
The scientists then characterized how the two phycotoxins bound to the receptor. Resolved using X-ray crystallography, the 3D structures of the complexes that formed between the phycotoxins and the receptor revealed that each toxin inserts itself at the heart of the binding site for acetylcholine, the natural neurotransmitter of this receptor. This is a key position in that it can block the channel receptor function of nRAChs. Of particular interest was the discovery that the binding mode of these toxins might provide new opportunities regarding the development of novel therapeutic agents active on nRAChs.
These results obtained in vitro thus explain the neurotoxicity of these phycotoxins in numerous animal species. A clearer understanding of their mode of action constitutes the first step towards the development of antidotes that might become a sanitary and economic necessity. Thus these findings raise hopes for the design of new, reliable, sensitive, practical and inexpensive tests that could detect the presence of phycotoxins in shellfish offered to consumers.
More information: Structural determinants in phycotoxins and AChBP conferring high affinity binding and nicotinic AChR antagonism. Yves Bourne, Zoran Radic, Romulo Aráoz, Todd T. Talley, Evelyne Benoit, Denis Servent, Palmer Taylor, Jordi Molgó, Pascale Marchot. Proc Natl Acad Sci USA, Online on PNAS website 8 March 2010.
Provided by CNRS