Metal oxide 'can transform'

(PhysOrg.com) -- A team including Oxford University scientists has been investigating what happens to the top layer of atoms on the surface of a material that splits water and has potential uses in nanoelectronics.
An international team, including Oxford University scientists, has been investigating what happens to the top layer of atoms on the surface of a material.
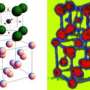
The material is strontium titanate: a complex metal oxide that many researchers are interested in because of its ability to split water into hydrogen and oxygen with sunlight and its potential for use in electronic devices.
The team used a variety of techniques including scanning tunneling microscopy (STM) to directly ‘see’ the arrangement of surface atoms. Their observations, reported in this week’s Nature Materials, reveal a series of structures with a surprisingly close and orderly arrangement.
‘In most materials, when you create a surface, the top layer of atoms rearrange to different positions from those in the rest of the material. This rearrangement of atoms is usually locked into a particular configuration that will minimise the surface energy.’ said Dr Martin Castell of Oxford University’s Department of Materials, an author of the paper. ‘However, this is not the case for the surface of strontium titanate that we have been studying. This surface forms a whole family of different structures. Chemists would call these structures a homologous series - something that is routinely observed in the bulk of crystals, but not until now on the surface.’
These ‘transformations’ could prove very important to researchers hoping to use strontium titanate in order to build new kinds of nanoelectronic devices or to grow thin films.
The report also suggests that the techniques developed by the researchers could make it possible to predict the surface structures of other oxides.
'We have needed to use many different sophisticated experimental and theoretical approaches to solve this problem,' said Dr Castell. 'Our aim is to continue to work closely with our collaborators at Northwestern University in the US to solve related materials problems.'
The research was conducted by a team was led by Dr Martin Castell of Oxford University UK and Professor Laurence Marks and Professor Ken Poeppelmeier of Northwestern University, USA.
More information: A report of the research, ‘A homologous series of structures on the surface of SrTiO3 (110)’, is published in this week’s Nature Materials. (www.nature.com/nmat/journal/va … t/full/nmat2636.html)
Provided by Oxford University