January 28, 2010 report
Nanocables could lead to more powerful lithium-ion batteries
(PhysOrg.com) -- By itself, titanium dioxide (TiO2) is a very poor electrode. Electrons move very slowly through the material - so slowly, in fact, that it can take years to fill a millimeter-thick piece of TiO2. However, things change when the TiO2 is extremely thin: a 10-nm-thick piece of TiO2 can be filled with electrons in milliseconds. Inspired by this ability, scientists have recently investigated whether TiO2 could be useful for fabricating high-capacity batteries.
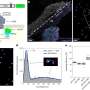
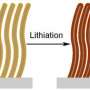
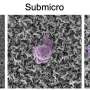
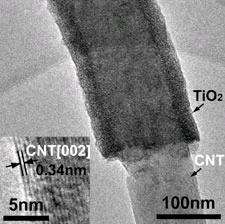
A team of scientists, Fei-Fei Cao, et al., from institutions in China and Germany, have found that applying a thin coat of TiO2 to the outside of carbon nanotubes (CNTs) can create coaxial nanocables. The nanocables can then be formed into a crystalline solid that turns out to be very good at trapping lithium ions and quickly transporting electrons - much better than either TiO2 or CNTs on their own.
“On one hand, the CNT core provides sufficient electrons for the storage of lithium in the TiO2 sheath,” the researchers wrote in a study published in Chemistry of Materials. “On the other hand, the CNT itself can also store lithium whereby this storage kinetics is, in turn, improved by the presence of the nanoporous TiO2 … [which] enables rapid access of lithium-ions from the liquid electrolyte.”
These symbiotic advantages could directly lead to improvements in lithium-ion batteries that use nanocable-based anodes. The researchers found the new anodes offer improvements in storage capacity, release rate, and cycling performance compared with either pure CNT or pure TiO2. The nanocables also had good reliability, showing almost no capacity loss after one hundred cycles.
These abilities are also competitive with graphite-based anodes, which are commonly used in today’s lithium-ion batteries. Plus, the nanocables are easy to produce and made of inexpensive materials, which could make them attractive for commercial use.
“This fascinating symbiotic behavior and the fact that the cable morphology leads to an efficient use of this symbiosis makes this solution match the requirements of lithium-ion batteries extremely well,” the researchers wrote.
The scientists hope that this demonstration of the synergistic benefits of hybrid materials might motivate further research in using hybrid materials for other energy storage devices, such as supercapacitors.
More information: Fei-Fei Cao, et al. "Symbiotic Coaxial Nanocables: Facile Synthesis and an Efficient and Elegant Morphological Solution to the Lithium Storage Problem." Chemistry of Materials. doi:10.1021/cm9036742
via: Chemistry World
© 2010 PhysOrg.com