Researchers image crucial anthrax protein
(PhysOrg.com) -- Anthrax, long feared for its potential as a biological weapon, has lost some of its mystery. Researchers at the U.S. Department of Energy's (DOE) Argonne National Laboratory, in collaboration with scientists at the University of Chicago, have determined the structure of a protein crucial to the virulence of anthrax bacteria.
One of anthrax’s most dangerous features is its ability to exist as a spore—an inactive sealed package that can survive extreme conditions outside its host for long periods. Once in the bloodstream, however, the bacterium germinates, triggering a dangerous infection. The activated anthrax bacterium looks like a thin rod swathed in a thick protective capsule coating. This capsule coat lets the bacteria evade macrophages, the roving white blood cells that form the first line of our immune response.
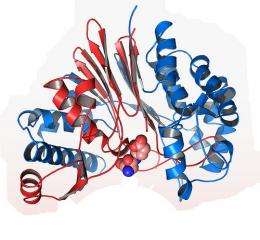
The researchers examined a gene that codes for a particular enzyme called CapD, a protein that helps the bacteria form its protective coating once it enters the body. CapD sits on the surface of the cell, grabs molecules of the capsule’s building blocks (poly-D-gamma-glutamic acid, or PDGA) and attaches them to the bacteria’s outer wall to form its shield.
“The PDGA peptide construction is unusual; for example, it is made with the ‘D’ isomer of glutamic acid instead of the ‘L’ form found in proteins,” said Andrzej Joachimiak, a co-author of the study. “This is why macrophages can’t deal with the protective capsule of anthrax. They could easily find a regular peptide made of ‘L’ amino acids, but this is a unique configuration, and they can’t recognize it. That’s how they fool the system.”
Joachimiak and the team wanted to map out the crystal structure of CapD. Once the researchers discovered the enzyme's structure, they could begin to search for a molecule that could block its function—which in turn would make the bacteria more vulnerable to human defenses.
By using brilliant X-rays at the Structural Biology Center 19ID beamline at Argonne's Advanced Photon Source, the team was able to image two versions of the protein: one with a molecule of imitation capsule material attached, and one without.
“It looks like Pac-Man,” Joachimiak said. “The jaws are open when it’s empty, but when the ligand attaches, it clamps down around it.” Then the enzyme cleaves the polymer and attaches it to the surface of the cell.
The paper, “Crystal Structure of Bacillus anthracis Transpeptidase Enzyme CapD”, will be published in the Journal of Biological Chemistry.
The work to characterize anthrax and other pathogenic or important bacteria is part of an initiative by the Midwest Center for Structural Genomics, an NIH-funded consortium of eight institutions, and the Region V Great Lakes Regional Center of Excellence in Biodefense and Emerging Infectious Diseases Consortium.
Provided by Argonne National Laboratory (news : web)


















